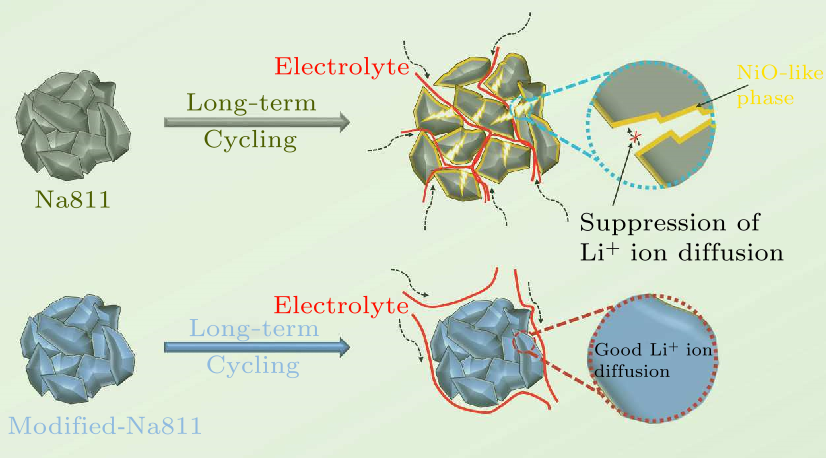
Fig. 1. The schematic illustration of degradation and modification for Na811.
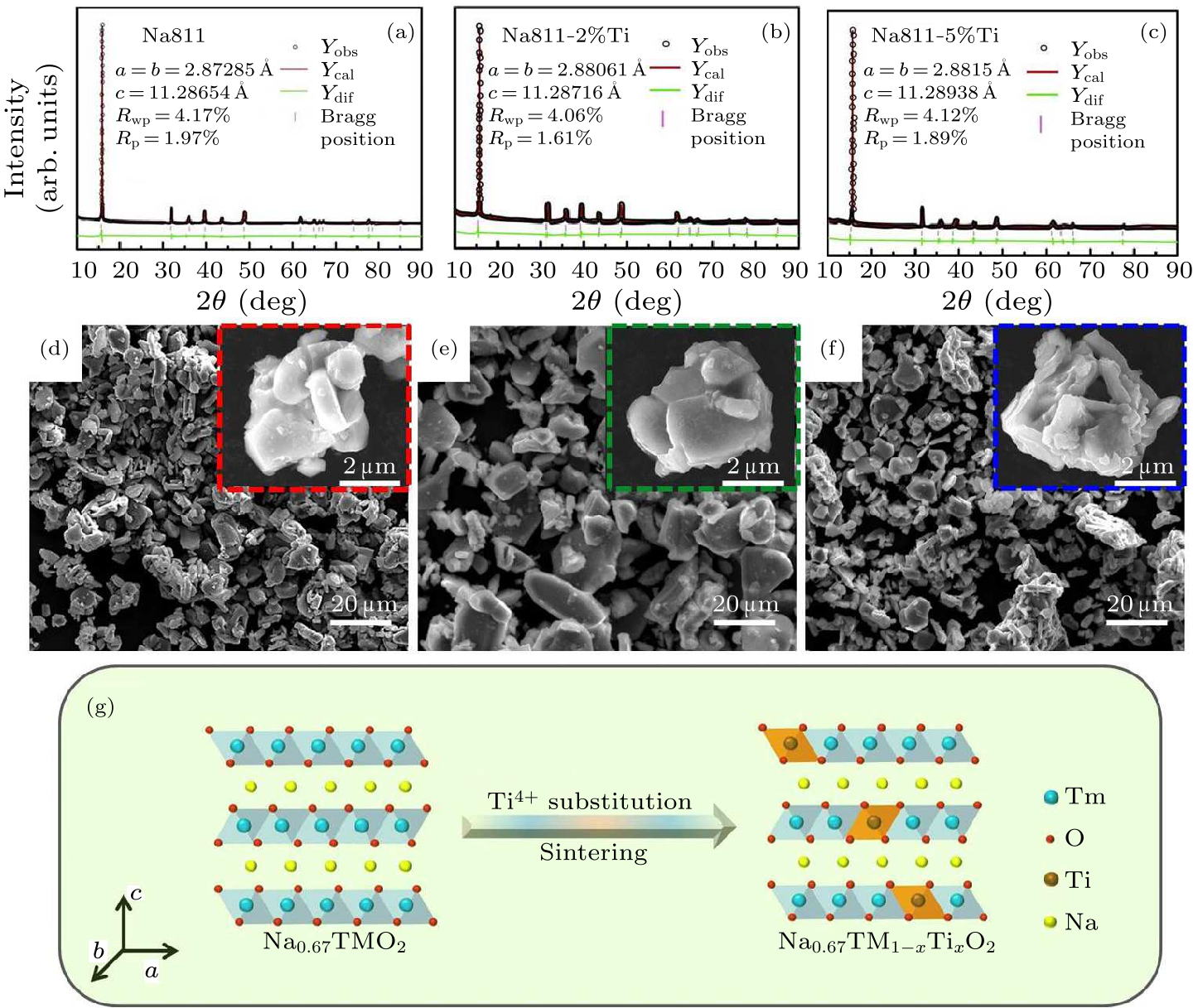
Fig. 2. x-ray diffraction (XRD) pattern and Rietveld plots of the samples: (a) Na811, (b) Na811-2%Ti, (c) Na811-5%Ti. Scanning electron microscopy (SEM) images of the samples: (d) Na811, (e) Na811-2%Ti, (f) Na811-5%Ti. (g) Structural change of Na811 with Ti doped. $R_{\rm wp}$ and $R_{\rm p}$ are the factors to evaluate the precision of refinement. $R_{\rm wp}$: weighted profile $R$-factor; $R_{\rm p}$: profile residual (unweighted).
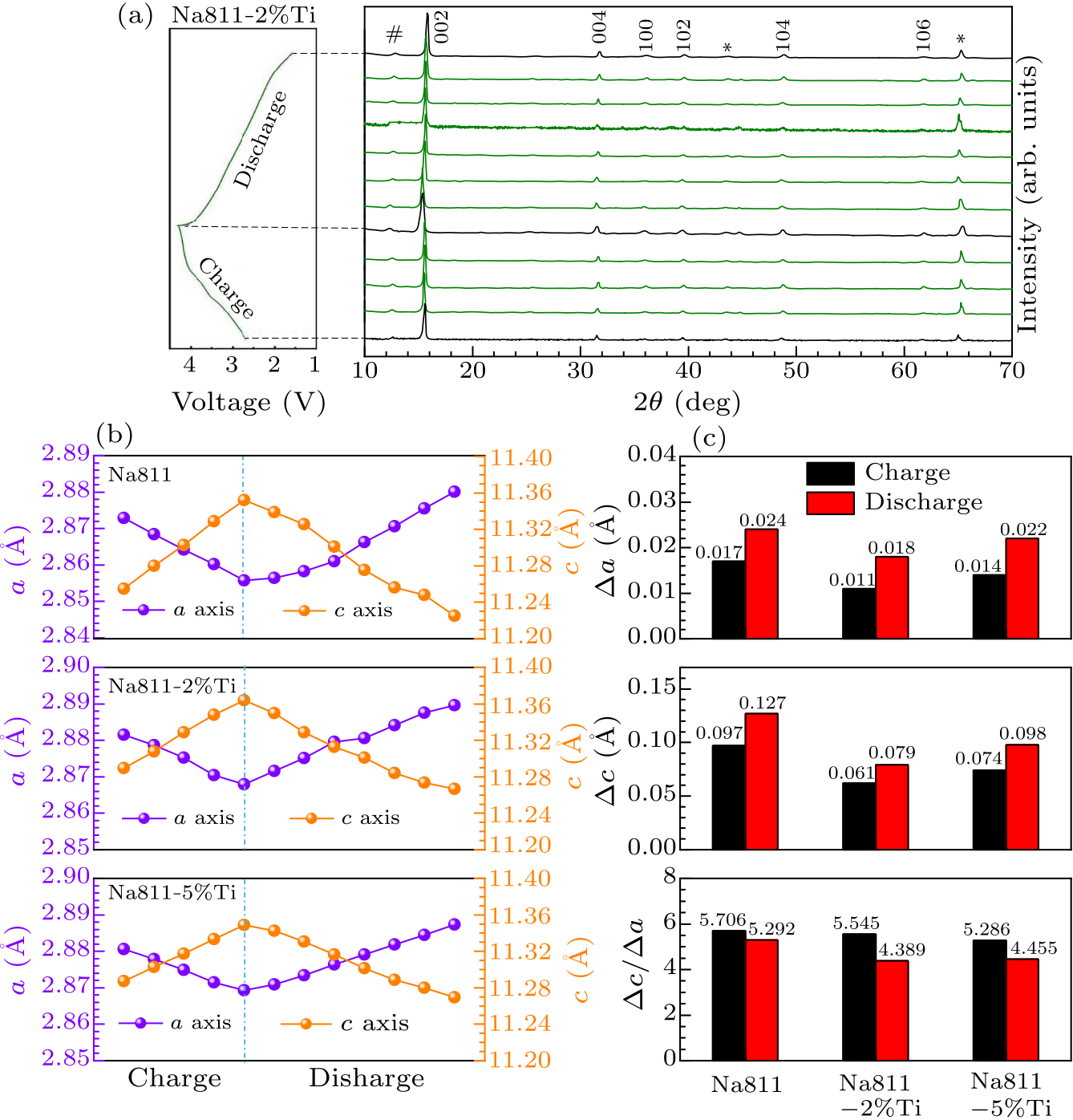
Fig. 3. (a) In situ XRD characterization of Na811-2%Ti; (b) variation of lattice $c$ on all investigated samples during 1$^{\rm st}$ charge and discharge; (c) $\Delta a$ and $\Delta c$ of all investigated during charge and discharge.
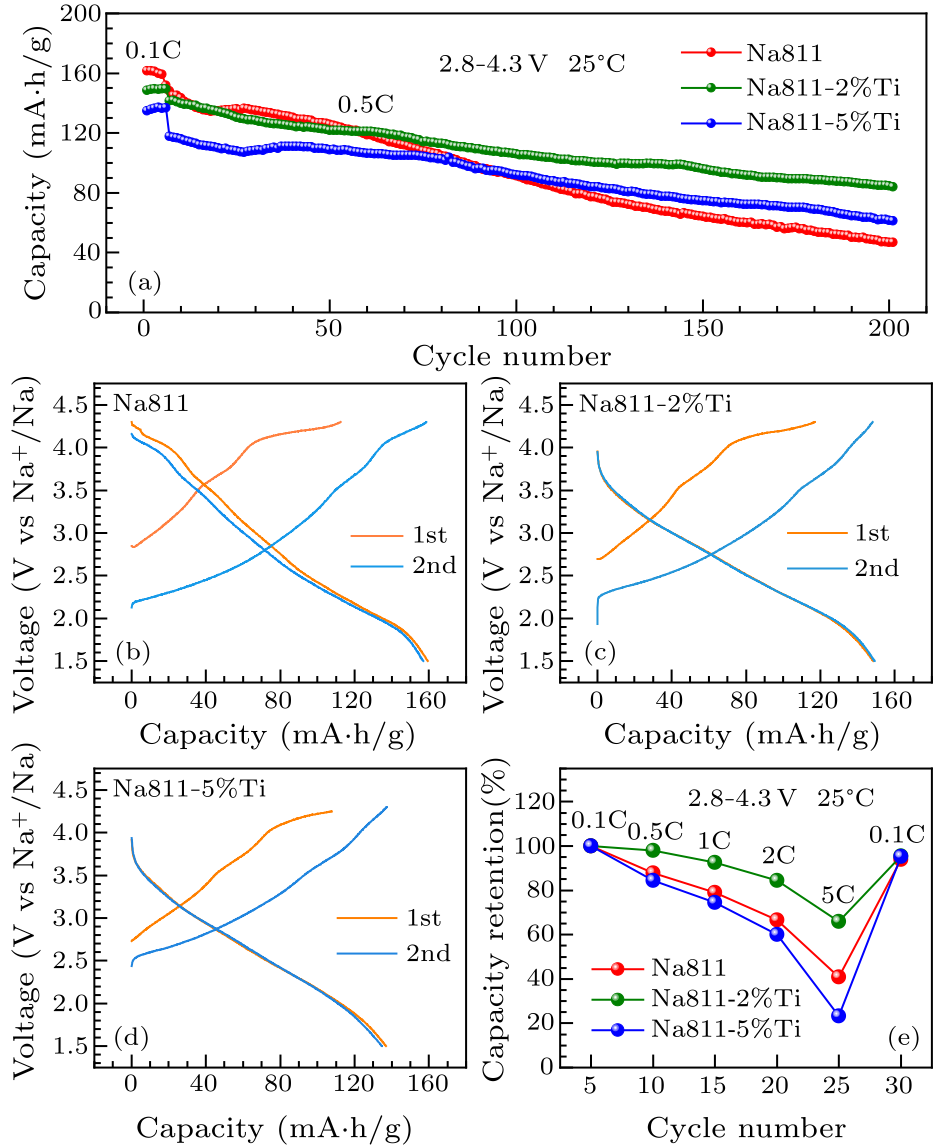
Fig. 4. (a) Cyclic performance of Na811, Na811-2%Ti and Na811-5%Ti. Charge and discharge profiles at selected cycles at 0.1 C (1$^{\rm st}$ and 2$^{\rm nd}$): (b) Na811, (c) Na811-2%Ti, (d) Na811-5%Ti. (e) Rate retention of Na811, Na811-2%Ti and Na811-5%Ti.
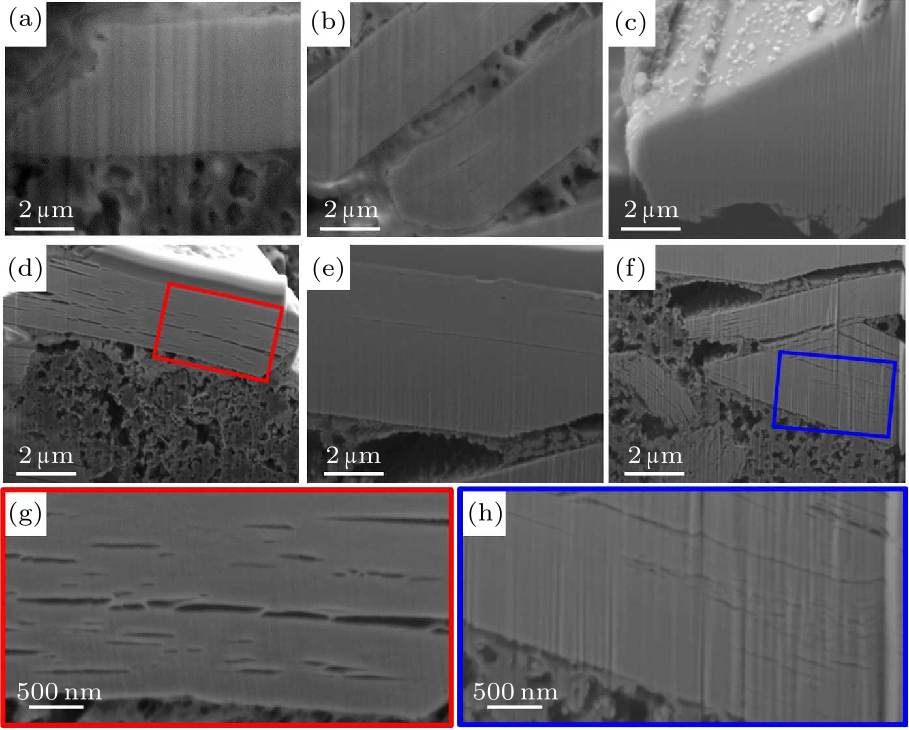
Fig. 5. Cross-sectional SEM of the samples before cycling: (a) Na811, (b) Na811-2%Ti, (c) Na811-5%Ti. Cross-sectional SEM of the samples after 200 charge/discharge cycles, measured at the current rate of 0.5 C: [(d), (g)] Na811, (e) Na811-2%Ti, [(f), (h)] Na811-5%Ti.
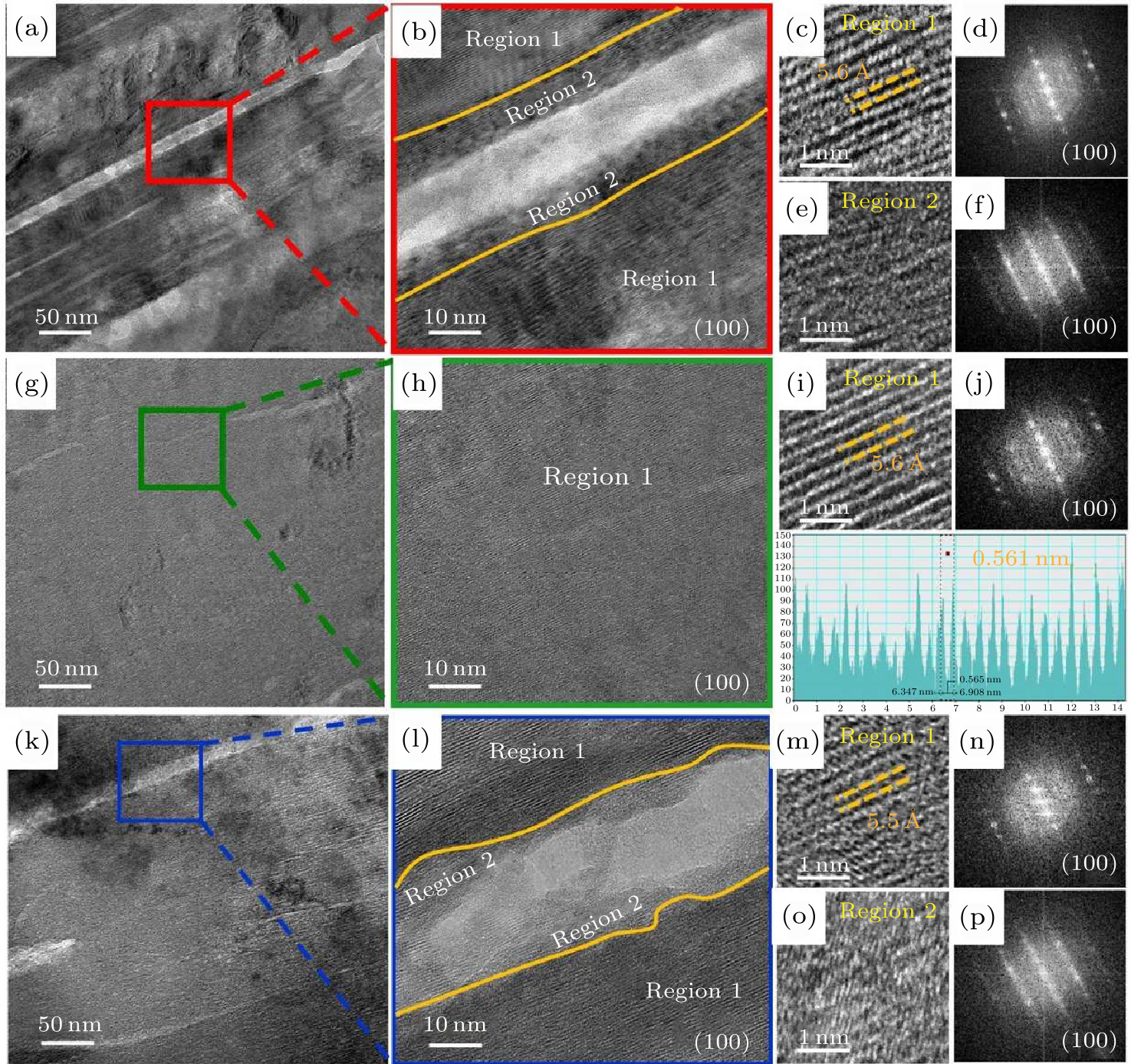
Fig. 6. [(a), (b), (c), (e), (g), (h), (i) & (k), (l), (m), (n)] TEM images of Na811, Na811-2%Ti & Na811-5%Ti cathodes after 200 charge/discharge cycles, measured at the current rates of 0.5 C. [(d), (f), (j) & (n), (p)] The corresponding FFT images of [(c), (e), (i) & (m), (o)].
| [1] | Yabuuchi N, Kubota K, Dahbi M, and Komaba S 2014 Chem. Rev. 114 11636 | Research Development on Sodium-Ion Batteries
| [2] | Kundu D, Talaie E, Duffort V, and Nazar L F 2015 Angew. Chem. Int. Ed. 54 3431 | The Emerging Chemistry of Sodium Ion Batteries for Electrochemical Energy Storage
| [3] | Liu W, Oh P, Liu X, Lee M J, Cho W, Chae S, Kim Y, and Cho J 2015 Angew. Chem. Int. Ed. 54 4440 | Nickel-Rich Layered Lithium Transition-Metal Oxide for High-Energy Lithium-Ion Batteries
| [4] | Kim J, Lee H, Cha H, Yoon M, Park M, and Cho J 2018 Adv. Energy Mater. 8 1702028 | Prospect and Reality of Ni-Rich Cathode for Commercialization
| [5] | Thackeray M M, Wolverton C, and Isaacs E D 2012 Energy & Environ. Sci. 5 7854 | Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium-ion batteries
| [6] | Li H S, Ding Y, Ha H, Shi Y, Peng L L, Zhang X G, Ellison C J, and Yu G H 2017 Adv. Mater. 29 1700898 | An All-Stretchable-Component Sodium-Ion Full Battery
| [7] | Wang P F, You Y, Yin Y X, Wang Y S, Wan L J, Gu L, and Guo Y G 2016 Angew. Chem. Int. Ed. 55 7445 | Suppressing the P2-O2 Phase Transition of Na 0.67 Mn 0.67 Ni 0.33 O 2 by Magnesium Substitution for Improved Sodium-Ion Batteries
| [8] | Yoon H J, Na R K, Jin H J, and Yun Y S 2018 Adv. Energy Mater. 8 1870027 | Sodium-Ion Batteries: Macroporous Catalytic Carbon Nanotemplates for Sodium Metal Anodes (Adv. Energy Mater. 6/2018)
| [9] | Xiong X, Ding D, Bu Y, Wang Z, Huang B, Guo H, and Li X 2014 J. Mater. Chem. A 2 11691 | Enhanced electrochemical properties of a LiNiO 2 -based cathode material by removing lithium residues with (NH 4 ) 2 HPO 4
| [10] | Sun Y K, Myung S T, Park B C, Prakash J, Belharouak I, and Amine K 2009 Nat. Mater. 8 320 | High-energy cathode material for long-life and safe lithium batteries
| [11] | Jung Y S, Lu P, Cavanagh A S, Ban C, Kim G H, Lee S H, George S M, Harris S J, and Dillon A C 2013 Adv. Energy Mater. 3 213 | Unexpected Improved Performance of ALD Coated LiCoO 2 /Graphite Li-Ion Batteries
| [12] | Choi N S, Chen Z, Freunberger S A et al. 2012 Angew. Chem. Int. Ed. 51 9994 | Challenges Facing Lithium Batteries and Electrical Double-Layer Capacitors
| [13] | Jung S K, Gwon H, Hong J, Park K Y, Seo D H, Kim H, Hyun J, Yang W, and Kang K 2014 Adv. Energy Mater. 4 1300787 | Understanding the Degradation Mechanisms of LiNi 0.5 Co 0.2 Mn 0.3 O 2 Cathode Material in Lithium Ion Batteries
| [14] | Scrosati B and Garche J 2010 J. Power Sources 195 2419 | Lithium batteries: Status, prospects and future
| [15] | Lee S W, Kim M S, Jeong J H et al. 2017 J. Power Sources 360 206 | Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: Improved thermal stability and high-voltage performance
| [16] | Yoon W S, Hanson J, McBreen J, and Yang X Q 2006 Electrochem. Commun. 8 859 | A study on the newly observed intermediate structures during the thermal decomposition of nickel-based layered cathode materials using time-resolved XRD
| [17] | Oh P, Song B, Li W, and Manthiram A 2016 J. Mater. Chem. A 4 5839 | Overcoming the chemical instability on exposure to air of Ni-rich layered oxide cathodes by coating with spinel LiMn 1.9 Al 0.1 O 4
| [18] | Cho Y, Oh P, and Cho J 2013 Nano Lett. 13 1145 | A New Type of Protective Surface Layer for High-Capacity Ni-Based Cathode Materials: Nanoscaled Surface Pillaring Layer
| [19] | Hwang J Y, Kim J, Yu T Y, and Sun Y K 2019 Adv. Energy Mater. 9 1803346 | A New P2‐Type Layered Oxide Cathode with Extremely High Energy Density for Sodium‐Ion Batteries
| [20] | Zhang X, Guo S, Liu P, Li Q, Xu S, Liu Y, Jiang K, He P, Chen M, Wang P, and Zhou H 2019 Adv. Energy Mater. 9 1900189 | Capturing Reversible Cation Migration in Layered Structure Materials for Na‐Ion Batteries
| [21] | Wang Q, Mariyappan S, Vergnet J, Abakumov A M, Rousse G, Rabuel F, Chakir M, and Tarascon J M 2019 Adv. Energy Mater. 9 1901785 | Reaching the Energy Density Limit of Layered O3‐NaNi 0.5 Mn 0.5 O 2 Electrodes via Dual Cu and Ti Substitution
| [22] | Zhu Y E, Qi X G, Chen X Q, Zhou X L, Zhang X, Wei J P, Hu Y S, and Zhou Z 2016 J. Mater. Chem. A 4 11103 | A P2-Na 0.67 Co 0.5 Mn 0.5 O 2 cathode material with excellent rate capability and cycling stability for sodium ion batteries
| [23] | Xu J L, Han Z, Jiang K, Bai P, Liang Y, Zhang X, Wang P, Guo S, and Zhou H 2020 Small 16 1904388 | Suppressing Cation Migration and Reducing Particle Cracks in a Layered Fe‐Based Cathode for Advanced Sodium‐Ion Batteries
| [24] | Wang K, Wan H, Yan P, Chen X, Fu J, Liu Z, Deng H, Gao F, and Sui M 2019 Adv. Mater. 31 1904816 | Dopant Segregation Boosting High‐Voltage Cyclability of Layered Cathode for Sodium Ion Batteries
| [25] | Wang Q C, Meng J K, Yue X Y, Qiu Q Q, Song Y, Wu X J, Fu Z W, Xia Y Y, Shadike Z, Wu J, Yang X Q, and Zhou Y N 2019 J. Am. Chem. Soc. 141 840 | Tuning P2-Structured Cathode Material by Na-Site Mg Substitution for Na-Ion Batteries
| [26] | Zhang K, Kim D, Hu Z, Park M, Noh G, Yang Y, Zhang J, Lau V W, Chou S L, Cho M, Choi S Y, and Kang Y M 2019 Nat. Commun. 10 5203 | Manganese based layered oxides with modulated electronic and thermodynamic properties for sodium ion batteries
| [27] | Hu X, Qiang W, and Huang B 2017 Energy Storage Mater. 8 141 | Surface layer design of cathode materials based on mechanical stability towards long cycle life for lithium secondary batteries
| [28] | Cheng X, Liu M, Yin J, Ma C, Dai Y, Wang D, Mi S, Qiang W, Huang B, and Chen Y 2020 Small 16 1906433 | Regulating Surface and Grain‐Boundary Structures of Ni‐Rich Layered Cathodes for Ultrahigh Cycle Stability
| [29] | Ren Z, Zhang X, Liu M, Zhou J, Sun S, He H, and Wang D 2019 J. Power Sources 416 104 | Constant dripping wears away a stone: Fatigue damage causing particles' cracking
| [30] | Ren Z, Shen C, Liu M, Liu J, Zhang S, Yang G, Huai L, Liu X, Wang D, and Li H 2020 Energy Storage Mater. 28 1 | Improving LiNi0.9Co0.08Mn0.02O2’s cyclic stability via abating mechanical damages
| [31] | Wang K, Yan P, and Sui M 2018 Nano Energy 54 148 | Phase transition induced cracking plaguing layered cathode for sodium-ion battery
| [32] | Yan P, Zheng J, Chen T, Luo L, Jiang Y, Wang K, Sui M, Zhang J G, Zhang S, and Wang C 2018 Nat. Commun. 9 2437 | Coupling of electrochemically triggered thermal and mechanical effects to aggravate failure in a layered cathode
| [33] | Yan P, Zheng J, Gu M, Xiao J, Zhang J G, and Wang C M 2017 Nat. Commun. 8 14101 | Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries
| [34] | Xu Z, Rahman M M, Mu L, Liu Y, and Lin F 2018 J. Mater. Chem. A 6 21859 | Chemomechanical behaviors of layered cathode materials in alkali metal ion batteries
| [35] | Guo S H, Liu P, Yu H J, Zhu Y B, Chen M W, Ishida M, and Zhou H S 2015 Angew. Chem. Int. Ed. 54 5894 | A Layered P2- and O3-Type Composite as a High-Energy Cathode for Rechargeable Sodium-Ion Batteries
| [36] | Mu L Q, Xu S Y, Li M, Hu Y S, Li H, Chen L Q, and Huang X J 2015 Adv. Mater. 27 6928 | Prototype Sodium-Ion Batteries Using an Air-Stable and Co/Ni-Free O3-Layered Metal Oxide Cathode
| [37] | Singh G, Tapia-Ruiz N, Lopez D A J M, Maitra U, Somerville J W, Armstrong A R, de Martinez I J, Rojo T, and Bruce P G 2016 Chem. Mater. 28 5087 | High Voltage Mg-Doped Na 0.67 Ni 0.3– x Mg x Mn 0.7 O 2 ( x = 0.05, 0.1) Na-Ion Cathodes with Enhanced Stability and Rate Capability
| [38] | Wang P, Xiao Y, Piao N, Wang Q, Ji X, Jin T, Guo Y, Liu S, Deng T, Cui C, Chen L, Guo Y, Yang X, and Wang C 2020 Nano Energy 69 104474 | Both cationic and anionic redox chemistry in a P2-type sodium layered oxide
| [39] | Liu K, Tan S, Moon J, Jafta C J, Li C, Kobayashi T, Lyu H, Bridges C A, Men S, Guo W, Sun Y, Zhang J, Paranthaman M P, Sun X, and Dai S 2020 Adv. Energy Mater. 10 2000135 | Insights into the Enhanced Cycle and Rate Performances of the F‐Substituted P2‐Type Oxide Cathodes for Sodium‐Ion Batteries
| [40] | Xiao B W, Liu H S, Liu J et al. 2017 Adv. Mater. 29 1703764 | Nanoscale Manipulation of Spinel Lithium Nickel Manganese Oxide Surface by Multisite Ti Occupation as High-Performance Cathode
| [41] | Yoon C S, Jun D W, Myung S T, and Sun Y K 2017 ACS Energy Lett. 2 1150 | Structural Stability of LiNiO 2 Cycled above 4.2 V
| [42] | Zhou Y N, Wang P F, Niu Y B, Li Q, Yu X, Yin Y X, Xu S, and Guo Y G 2019 Nano Energy 55 143 | A P2/P3 composite layered cathode for high-performance Na-ion full batteries
| [43] | Hwang J Y, Myung S T, and Sun Y K 2017 Chem. Soc. Rev. 46 3529 | Sodium-ion batteries: present and future
| [44] | Wang Y S, Yu X Q, Xu S Y, Bai J M, Xiao R J, Hu Y S, Li H, Yang X Q, Chen L Q, and Huang X J 2013 Nat. Commun. 4 2365 | A zero-strain layered metal oxide as the negative electrode for long-life sodium-ion batteries
| [45] | Yabuuchi N, Kajiyama M, Iwatate J, Nishikawa H, Hitomi S, Okuyama R, Usui R, Yamada Y, and Komaba S 2012 Nat. Mater. 11 512 | P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries
| [46] | Berthelot R, Carlier D, and Delmas C 2011 Nat. Mater. 10 74 | Electrochemical investigation of the P2–NaxCoO2 phase diagram
| [47] | Ryn H, Park K, Yoon C, and Sun Y 2018 Chem. Mater. 30 1155 | Capacity Fading of Ni-Rich Li[Ni x Co y Mn 1– x – y ]O 2 (0.6 ≤ x ≤ 0.95) Cathodes for High-Energy-Density Lithium-Ion Batteries: Bulk or Surface Degradation?