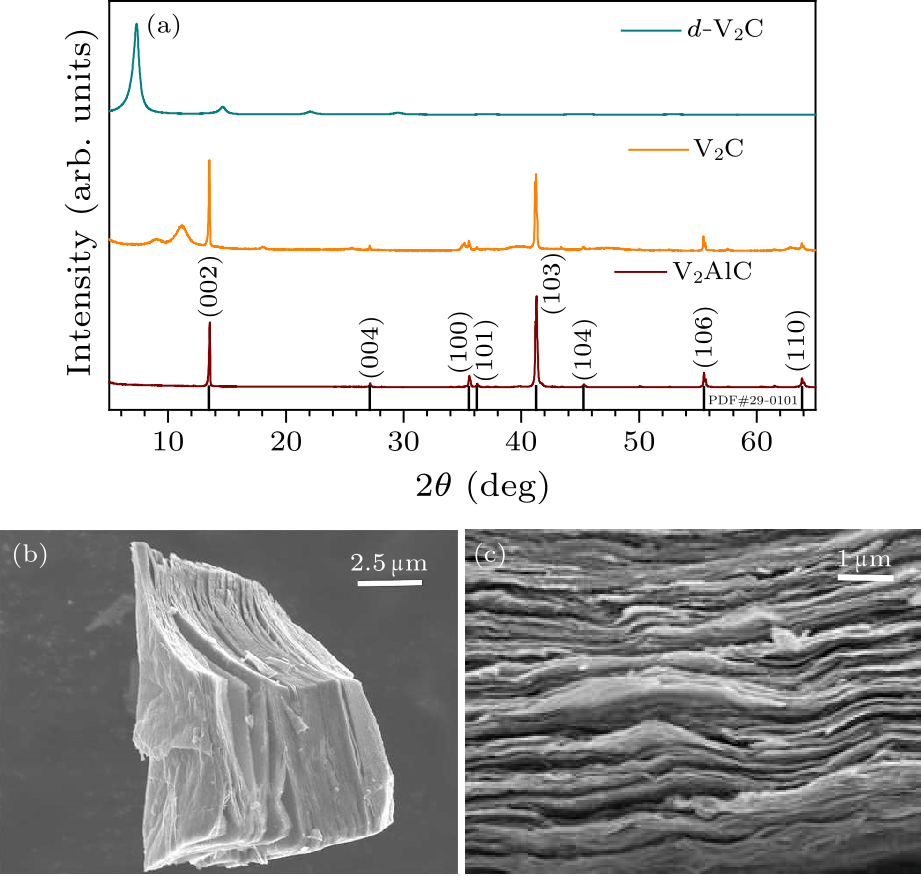
Fig. 1. (a) XRD patterns of $V_{2}$AlC, V$_{2}$C, and $d$-V$_{2}$C film after delamination. (b) SEM image of V$_{2}$C after etching. (c) SEM image of filtrated $d$-V$_{2}$C paper.

Fig. 2. Cyclic voltammograms of $d$-V$_{2}$C at different scan rates in (a) 0.5 M Li$_{2}$SO$_{4}$, (b) 0.5 M Na$_{2}$SO$_{4}$, (c) 0.5 M K$_{2}$SO$_{4}$, and (d) 1 M MgSO$_{4}$.
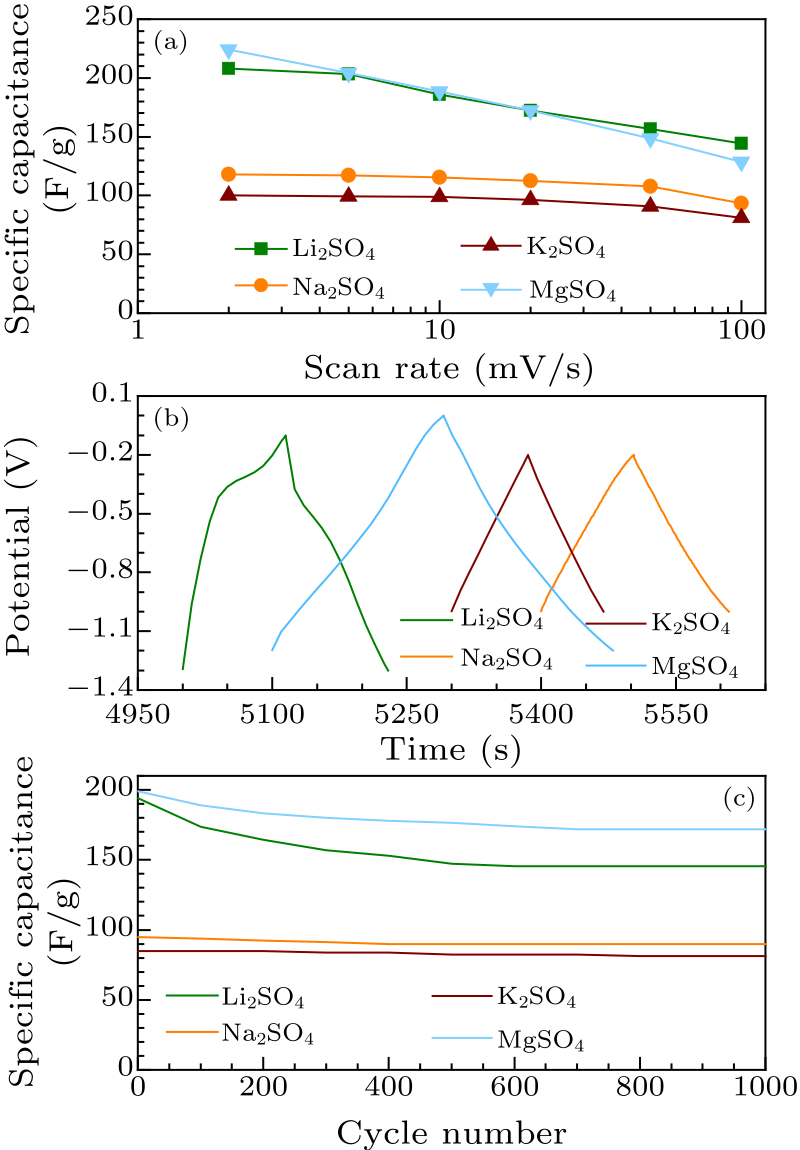
Fig. 3. Electrochemical performance of $d$-V$_{2}$C electrode. (a) Specific capacitance measured from corresponding $C$–$V$ patterns. (b) Galvanostatic charge and discharge profiles at 1 A/g for different electrolytes. (c) Cycle lives measured from galvanostatic charge to discharge at 1 A/g.
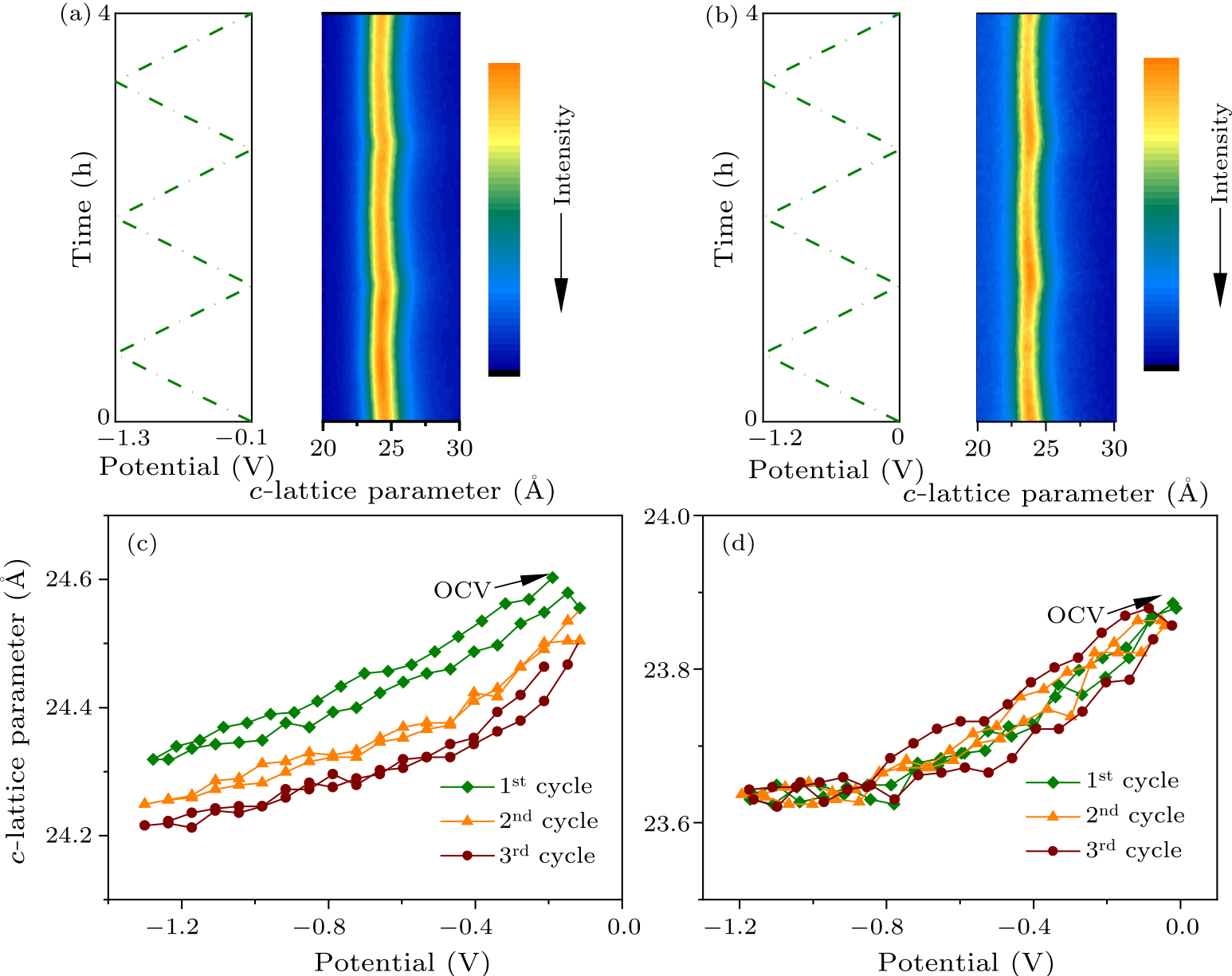
Fig. 4. In situ XRD patterns of $d$-V$_{2}$C during electrochemical cycles in (a) 0.5 M Li$_{2}$SO$_{4}$, and (b) 1 M MgSO$_{4}$. Change of the $c$-lattice parameter with potential during the first 3 cycles in (c) 0.5 M Li$_{2}$SO$_{4}$, and (d) 1 M MgSO$_{4}$.
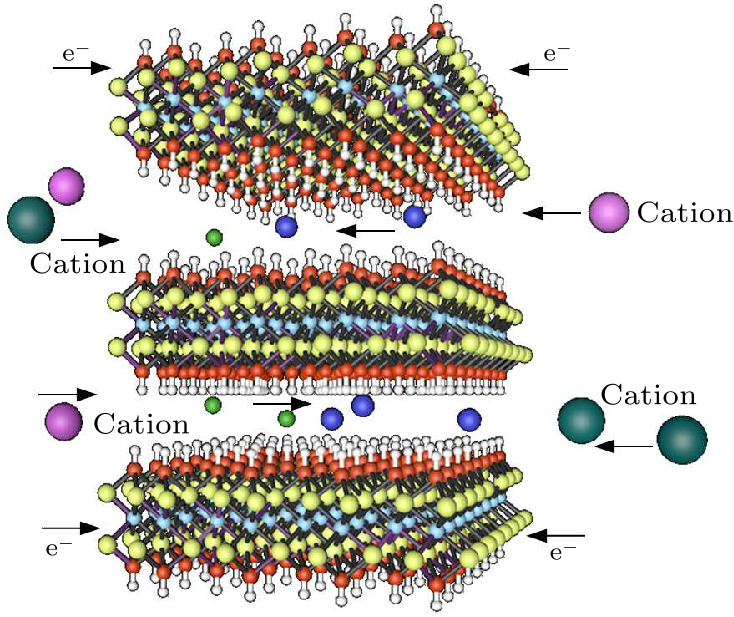
Fig. 5. Schematic illustration of V$_{2}$C during the discharge process. (yellow, V; sky blue, C; red, O; white, H; interlayer species: cations with different ion radius).
| [1] | Naguib M et al 2012 ACS Nano 6 1322 | Two-Dimensional Transition Metal Carbides
| [2] | Naguib M et al 2014 Adv. Mater. 26 992 | 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials
| [3] | Lukatskaya M R et al 2013 Science 341 1502 | Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide
| [4] | Come J et al 2012 J. Electrochem. Soc. 159 A1368 | A Non-Aqueous Asymmetric Cell with a Ti 2 C-Based Two-Dimensional Negative Electrode
| [5] | Zhao S S et al 2017 Energy Storage Mater. 8 42 | Li-ion uptake and increase in interlayer spacing of Nb4C3 MXene
| [6] | Mashtalir O et al 2015 Adv. Mater. 27 3501 | Amine-Assisted Delamination of Nb 2 C MXene for Li-Ion Energy Storage Devices
| [7] | Dall'Agnese Y et al 2015 J. Phys. Chem. Lett. 6 2305 | Two-Dimensional Vanadium Carbide (MXene) as Positive Electrode for Sodium-Ion Capacitors
| [8] | Naguib M et al 2012 Electrochem. Commun. 16 61 | MXene: a promising transition metal carbide anode for lithium-ion batteries
| [9] | Ghidiu M et al 2014 Nature 516 78 | Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance
| [10] | Wang X et al 2015 Nat. Commun. 6 6544 | Pseudocapacitance of MXene nanosheets for high-power sodium-ion hybrid capacitors
| [11] | Lukatskaya M R et al 2017 Nat. Energy 2 17105 | Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides
| [12] | Levi M D et al 2015 Adv. Energy Mater. 5 1400815 | Solving the Capacitive Paradox of 2D MXene using Electrochemical Quartz-Crystal Admittance and In Situ Electronic Conductance Measurements
| [13] | Lukatskaya M R et al 2015 Adv. Energy Mater. 5 1500589 | Probing the Mechanism of High Capacitance in 2D Titanium Carbide Using In Situ X-Ray Absorption Spectroscopy
| [14] | Lin Z F et al 2016 Electrochem. Commun. 72 50 | Electrochemical and in-situ X-ray diffraction studies of Ti 3 C 2 T x MXene in ionic liquid electrolyte
| [15] | Mu X P et al 2019 Adv. Funct. Mater. 29 1902953 | Revealing the Pseudo‐Intercalation Charge Storage Mechanism of MXenes in Acidic Electrolyte
| [16] | Wang X H et al 2019 Nat. Energy 4 241 | Influences from solvents on charge storage in titanium carbide MXenes
| [17] | Naguib M et al 2013 J. Am. Chem. Soc. 135 15966 | New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries
| [18] | Bak S M et al 2017 Adv. Energy Mater. 7 1700959 | Na-Ion Intercalation and Charge Storage Mechanism in 2D Vanadium Carbide
| [19] | Wang C D et al 2018 Adv. Mater. 30 1802525 | Atomic Cobalt Covalently Engineered Interlayers for Superior Lithium-Ion Storage
| [20] | Wang C D et al 2019 Small Methods 3 1900495 | Delaminating Vanadium Carbides for Zinc‐Ion Storage: Hydrate Precipitation and H + /Zn 2+ Co‐Action Mechanism
| [21] | VahidMohammadi A et al 2017 ACS Nano 11 11135 | Two-Dimensional Vanadium Carbide (MXene) as a High-Capacity Cathode Material for Rechargeable Aluminum Batteries
| [22] | Shan Q M et al 2018 Electrochem. Commun. 96 103 | Two-dimensional vanadium carbide (V2C) MXene as electrode for supercapacitors with aqueous electrolytes
| [23] | Mashtalir O et al 2013 Nat. Commun. 4 1716 | Intercalation and delamination of layered carbides and carbonitrides
| [24] | Simon P and Gogotsi Y 2008 Nat. Mater. 7 845 | Materials for electrochemical capacitors
| [25] | Wang G P et al 2012 Chem. Soc. Rev. 41 797 | A review of electrode materials for electrochemical supercapacitors
| [26] | Ghidiu M et al 2016 Chem. Mater. 28 3507 | Ion-Exchange and Cation Solvation Reactions in Ti 3 C 2 MXene
| [27] | Shi S Q et al 2016 Chin. Phys. B 25 018212 | Multi-scale computation methods: Their applications in lithium-ion battery research and development
| [28] | Ando Y et al 2020 Adv. Funct. Mater. 30 2000820 | Capacitive versus Pseudocapacitive Storage in MXene