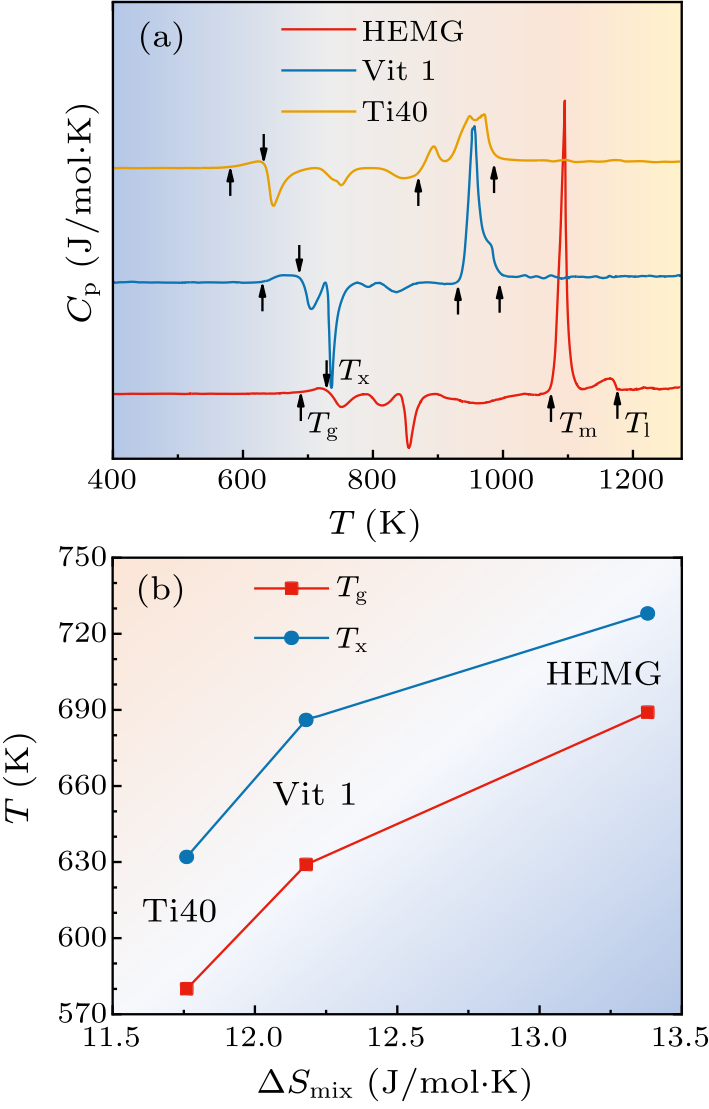

Fig. 1. (a) DSC curves of as-cast Zr$_{20}$Ti$_{20}$Cu$_{20}$Ni$_{20}$Be$_{20}$ (HEMG), Zr$_{41}$Ti$_{14}$Cu$_{12.5}$Ni$_{10}$Be$_{22.5}$ (Vit 1) and Ti$_{40}$Zr$_{25}$Cu$_{12}$Ni$_{3}$Be$_{20}$ (Ti40) metallic glasses. (b) The dependences of glass transition temperature $T_{\rm g}$ and initial crystallization temperature $T_{\rm x}$ on the mixing entropy $\Delta S_{\rm mix}$.
| $T_{\rm g}$ (K) | $T_{\rm x}$ (K) | $T_{\rm m}$ (K) | $T_{\rm l}$ (K) | $\Delta T$ | $T_{\rm rg}$ | $\gamma$ | |
|---|---|---|---|---|---|---|---|
| HEMG | 689 | 728 | 1073 | 1176 | 39 | 0.586 | 0.390 |
| Vit 1 | 629 | 686 | 930 | 995 | 57 | 0.632 | 0.422 |
| Ti40 | 580 | 632 | 870 | 986 | 52 | 0.588 | 0.403 |

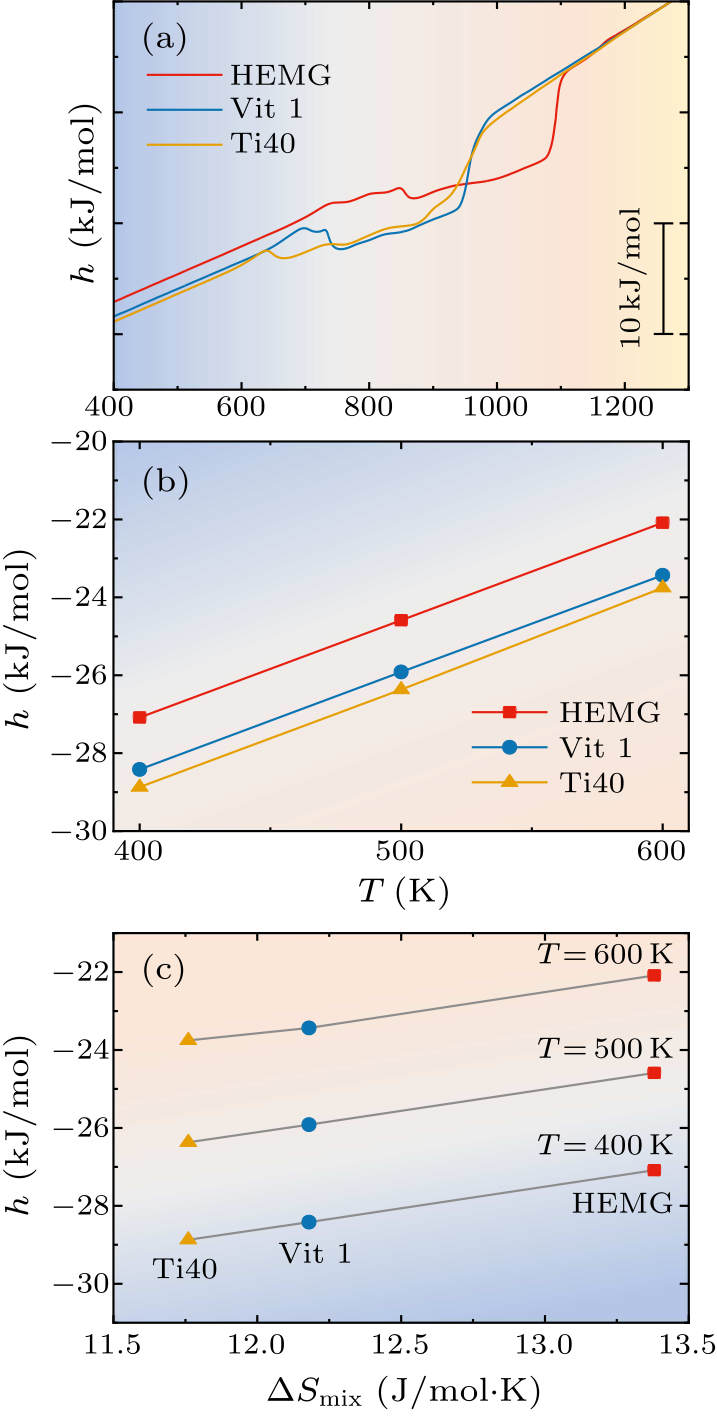
Fig. 2. (a) The relative enthalpy of HEMG, Vit1 and Ti40 metallic glasses. (b) The relative enthalpy of the three samples at three representative temperatures $T=400,\, 500$ and 600 K. (c) The relationship between enthalpy changes and mixing entropy at 400, 500 and 600 K, respectively.

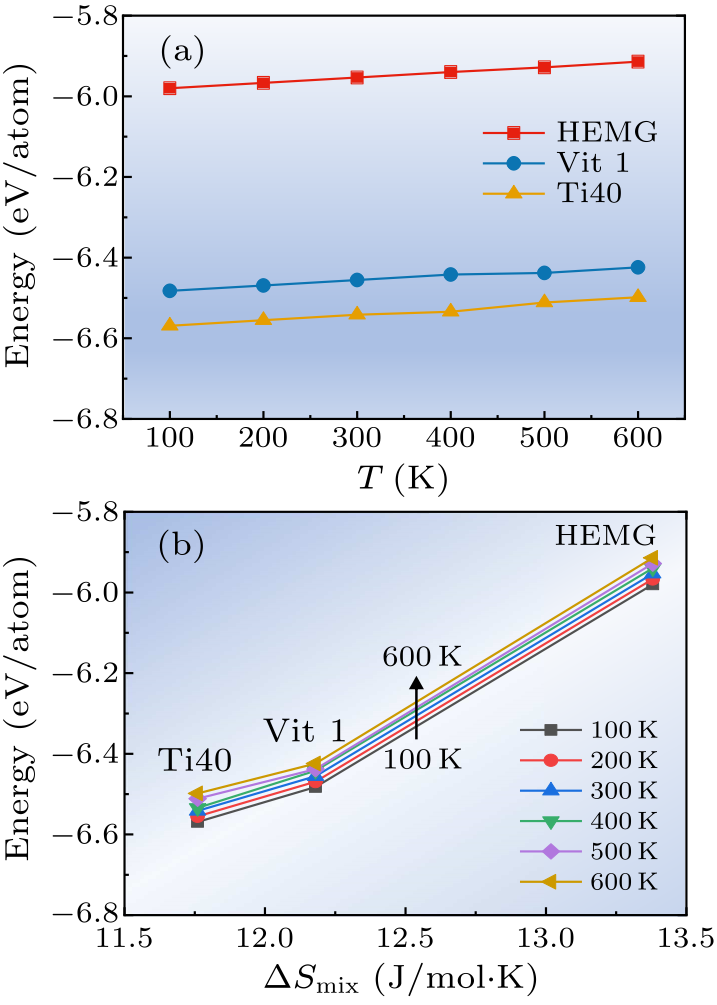
Fig. 3. (a) The energy per atom for HEMG, Vit1 and Ti40 metallic glasses at 100, 200, 300, 400, 500, and 600 K, respectively. (b) The dependence of energy on mixing entropy $\Delta S_{\rm mix}$.

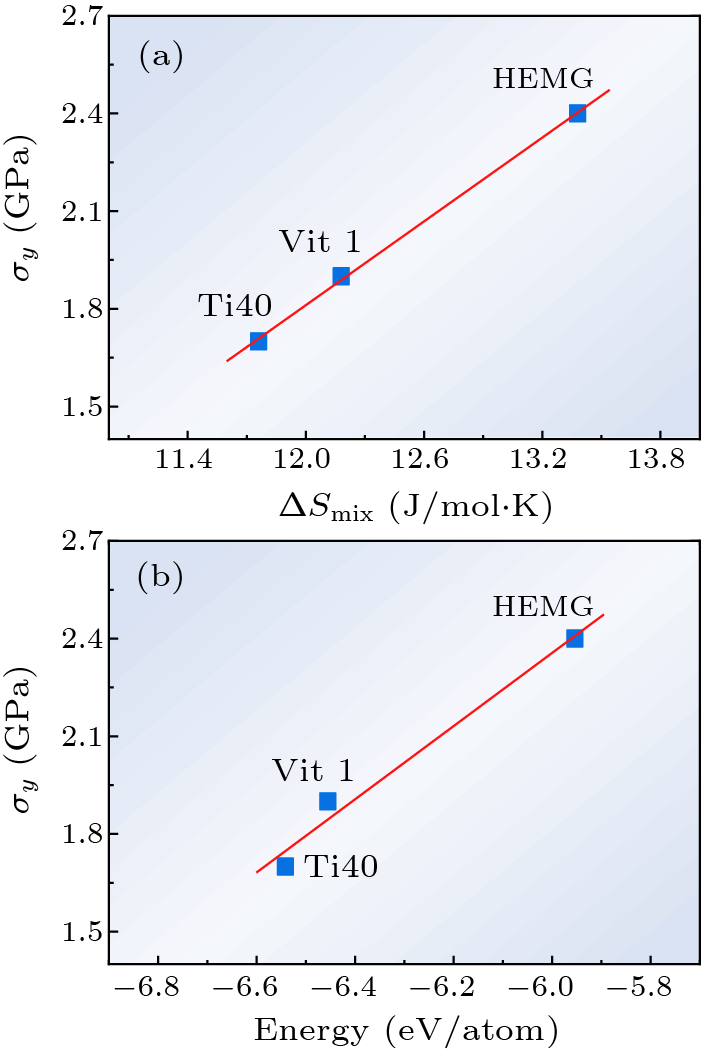
Fig. 4. The dependences of yield strength $\sigma_{y}$ on the (a) mixing entropy $\Delta S_{\rm mix}$, and (b) energy at 300 K.
| [1] | Wang W H 2009 Adv. Mater. 21 4524 | Bulk Metallic Glasses with Functional Physical Properties
| [2] | Schroers J 2010 Adv. Mater. 22 1566 | Processing of Bulk Metallic Glass
| [3] | Wang W H, Dong C, and Shek C H 2004 Mater. Sci. Eng. R 44 45 | Bulk metallic glasses
| [4] | Wang W H 2012 Prog. Mater. Sci. 57 487 | The elastic properties, elastic models and elastic perspectives of metallic glasses
| [5] | Li D M, Chen L S, Yu P, Ding D, and Xia L 2020 Chin. Phys. Lett. 37 086401 | A New Cu-Based Metallic Glass Composite with Excellent Mechanical Properties*
| [6] | Zhang S, Wang W, and Guan P 2021 Chin. Phys. Lett. 38 016802 | Dynamic Crossover in Metallic Glass Nanoparticles*
| [7] | Dong J, Feng Y H, Huan Y, Yi J, Wang W H, Bai H Y, and Sun B A 2020 Chin. Phys. Lett. 37 017103 | Rejuvenation in Hot-Drawn Micrometer Metallic Glassy Wires*
| [8] | Wang Y J, Du J P, Shinzato S, Dai L H, and Ogata S 2018 Acta Mater. 157 165 | A free energy landscape perspective on the nature of collective diffusion in amorphous solids
| [9] | Sun Y, Concustell A, and Greer A L 2016 Nat. Rev. Mater. 1 16039 | Thermomechanical processing of metallic glasses: extending the range of the glassy state
| [10] | Shen J, Huang Y J, and Sun J F 2007 J. Mater. Res. 22 3067 | Plasticity of a TiCu-based bulk metallic glass: Effect of cooling rate
| [11] | Xiao Y, Wu Y, Liu Z, Wu H, and Lue Z 2010 Sci. Chin. Phys. Mech. & Astron. 53 394 | Effects of cooling rates on the mechanical properties of a Ti-based bulk metallic glass
| [12] | Miyazaki N, Lo Y C, Wakeda M, and Ogata S 2016 Appl. Phys. Lett. 109 091906 | Properties of high-density, well-ordered, and high-energy metallic glass phase designed by pressurized quenching
| [13] | Wang W H 2019 Prog. Mater. Sci. 106 100561 | Dynamic relaxations and relaxation-property relationships in metallic glasses
| [14] | Ediger M D, Gruebele M, Lubchenko V, and Wolynes P G 2021 J. Phys. Chem. B 125 9052 | Glass Dynamics Deep in the Energy Landscape
| [15] | Tong X, Zhang Y, Wang Y, Liang X, Zhang K, Zhang F, Cai Y, Ke H, Wang G, Shen J, Makino A, and Wang W 2022 J. Mater. Sci. & Technol. 96 233 | Structural origin of magnetic softening in a Fe-based amorphous alloy upon annealing
| [16] | He N, Song L, Xu W, Huo J, Wang J Q, and Li R W 2019 J. Non-Cryst. Solids 509 95 | The evolution of relaxation modes during isothermal annealing and its influence on properties of Fe-based metallic glass
| [17] | Pan J and Duan F 2021 Acta Metall. Sin. 57 439 | Rejuvenation Behaviors in Metallic Glasses
| [18] | Jiang S, Huang Y, and Li M 2019 Chin. Phys. B 28 046103 | Structural evolution in deformation-induced rejuvenation in metallic glasses: A cavity perspective
| [19] | Qiang J and Tsuchiya K 2017 J. Alloys Compd. 712 250 | Composition dependence of mechanically-induced structural rejuvenation in Zr-Cu-Al-Ni metallic glasses
| [20] | Feng S D, Chan K C, Zhao L, Pan S P, Qi L, Wang L M, and Liu R P 2018 Mater. & Des. 158 248 | Rejuvenation by weakening the medium range order in Zr46Cu46Al8 metallic glass with pressure preloading: A molecular dynamics simulation study
| [21] | Guo W, Yamada R, Saida J, Lu S, and Wu S 2018 Nanoscale Res. Lett. 13 398 | Various Rejuvenation Behaviors of Zr-Based Metallic Glass by Cryogenic Cycling Treatment with Different Casting Temperatures
| [22] | Saida J, Yamada R, Wakeda M, and Ogata S 2017 Sci. Technol. Adv. Mater. 18 152 | Thermal rejuvenation in metallic glasses
| [23] | Miyazaki N, Wakeda M, Wang Y J, and Ogata S 2016 npj Comput. Mater. 2 16013 | Prediction of pressure-promoted thermal rejuvenation in metallic glasses
| [24] | Priezjev N V 2019 J. Mater. Res. 34 2664 | Potential energy states and mechanical properties of thermally cycled binary glasses
| [25] | Priezjev N V 2019 J. Non-Cryst. Solids 503 131 | The effect of cryogenic thermal cycling on aging, rejuvenation, and mechanical properties of metallic glasses
| [26] | Ma Y B, Mei L, Cui X, and Zu F Q 2021 Kovove Mater.-Metallic Mater. 59 181 | Improved tensile plasticity of bulk metallic glasses by heightening microstructural heterogeneity and energy state
| [27] | Ri M C, Sohrabi S, Ding D W, Dong B S, Zhou S X, and Wang W H 2017 Chin. Phys. B 26 066101 | Serrated magnetic properties in metallic glass by thermal cycle
| [28] | Afonin G V, Mitrofanov Y P, Kobelev N P, Pinto M W D S, Wilde G, and Khonik V A 2019 Scr. Mater. 166 6 | Relationship between the enthalpies of structural relaxation, crystallization and melting in metallic glass-forming systems
| [29] | Wang J G, Yang H, Pan Y, Song Y J, Li W H, and He Y Z 2016 J. Non-Cryst. Solids 452 273 | Structure transformation and fractography in Zr20Ti20Cu20Ni20Be20 metallic glass
| [30] | Guo F Q, Wang H J, Poon S J, and Shiflet G J 2005 Appl. Phys. Lett. 86 091907 | Ductile titanium-based glassy alloy ingots
| [31] | Lu J, Ravichandran G, and Johnson W L 2003 Acta Mater. 51 3429 | Deformation behavior of the Zr41.2Ti13.8Cu12.5Ni10Be22.5 bulk metallic glass over a wide range of strain-rates and temperatures
| [32] | Kresse G and Furthmuller J 1996 Phys. Rev. B 54 11169 | Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set
| [33] | Sheng H W, Luo W K, Alamgir F M, Bai J M, and Ma E 2006 Nature 439 419 | Atomic packing and short-to-medium-range order in metallic glasses
| [34] | Blöchl P E 1994 Phys. Rev. B 50 17953 | Projector augmented-wave method
| [35] | Wang Y and Perdew J P 1991 Phys. Rev. B 44 13298 | Correlation hole of the spin-polarized electron gas, with exact small-wave-vector and high-density scaling
| [36] | Pinal R 2008 Entropy 10 207 | Entropy of Mixing and the Glass Transition of Amorphous Mixtures
| [37] | Inaba S, Oda S, and Morinaga K 2003 J. Non-Cryst. Solids 325 258 | Heat capacity of oxide glasses at high temperature region
| [38] | Ke H B, Wen P, Zhao D Q, and Wang W H 2010 Appl. Phys. Lett. 96 251902 | Correlation between dynamic flow and thermodynamic glass transition in metallic glasses
| [39] | Höhne G, McNaughton J, Hemminger W, Flammersheim H J, and Flammersheim H J 2003 Differential Scanning Calorimetry 2nd edn (New York: Springer Science & Business Media) p 147 |