| CROSS-DISCIPLINARY PHYSICS AND RELATED AREAS OF SCIENCE AND TECHNOLOGY |
 |
|
|
|
|
Fluorination Increases Hydrophobicity at the Macroscopic Level but not at the Microscopic Level |
| Weishuai Di1, Xin Wang1, Yanyan Zhou1, Yuehai Mei2, Wei Wang2,3,4*, and Yi Cao1,2,3,4* |
1Oujiang Laboratory (Zhejiang Lab for Regenerative Medicine, Vision and Brain Health), Wenzhou Institute, University of Chinese Academy of Sciences, Wenzhou 325001, China
2Collaborative Innovation Center of Advanced Microstructures, National Laboratory of Solid State Microstructure, Department of Physics, Nanjing University, Nanjing 210093, China
3Institute for Brain Sciences, Nanjing University, Nanjing 210093, China
4Chemistry and Biomedicine Innovation Center, Nanjing University, Nanjing 210093, China
|
|
| Cite this article: |
|
Weishuai Di, Xin Wang, Yanyan Zhou et al 2022 Chin. Phys. Lett. 39 038701 |
|
|
|
|
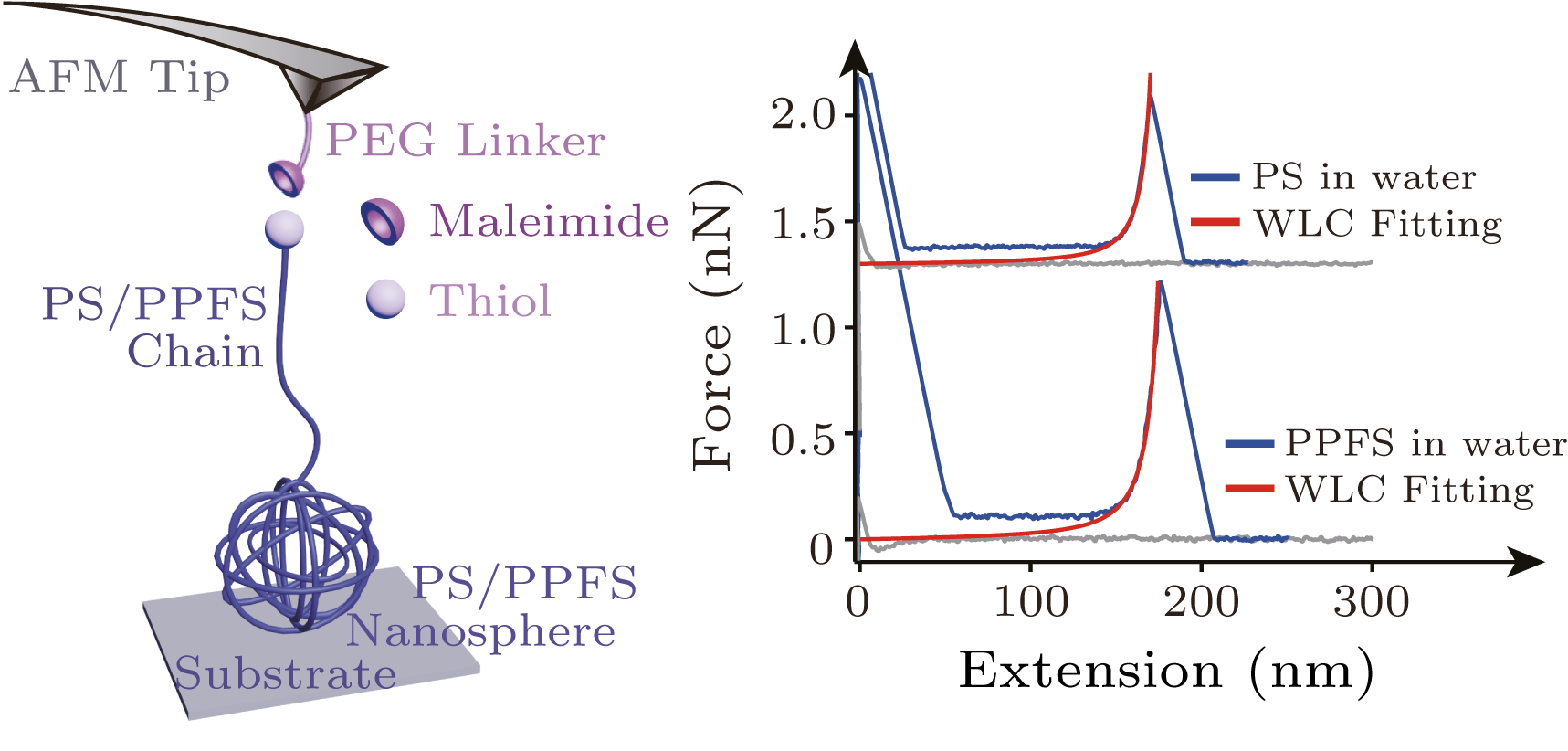
Abstract Hydrophobic interactions have been studied before in detail based on hydrophobic polymers, such as polystyrene (PS). Because fluorinated materials have relatively low surface energy, they often show both oleophobicity and hydrophobicity at the macroscopic level. However, it remains unknown how fluorination of hydrophobic polymer influences hydrophobicity at the microscopic level. We synthesized PS and fluorine-substituted PS (FPS) by employing the reversible addition-fragmentation chain transfer polymerization method. Contact angle measurements confirmed that FPS is more hydrophobic than PS at the macroscopic level due to the introduction of fluorine. However, single molecule force spectroscopy experiments showed that the forces required to unfold the PS and FPS nanoparticles in water are indistinguishable, indicating that the strength of the hydrophobic effect that drives the self-assembly of PS and FPS nanoparticles is the same at the microscopic level. The divergence of hydrophobic effect at the macroscopic and microscopic level may hint different underlying mechanisms: the hydrophobicity is dominated by the solvent hydration at the microscopic level and the surface-associated interaction at the macroscopic level.
|
|
Received: 29 December 2021
Express Letter
Published: 31 January 2022
|
|
| PACS: |
87.80.Nj
|
(Single-molecule techniques)
|
| |
82.70.Uv
|
(Surfactants, micellar solutions, vesicles, lamellae, amphiphilic systems, (hydrophilic and hydrophobic interactions))
|
| |
87.64.-t
|
(Spectroscopic and microscopic techniques in biophysics and medical physics)
|
|
|
|
|
|
| [1] | Koldewey P, Stull F, Horowitz S, Martin R, and Bardwell J C A 2016 Cell 166 369 |
| [2] | Kumar A, Singh N K, Ghosh D, and Radhakrishna M 2021 Phys. Chem. Chem. Phys. 23 12620 |
| [3] | Berne B J, Weeks J D, and Zhou R 2009 Annu. Rev. Phys. Chem. 60 85 |
| [4] | Jamadagni S N, Godawat R, and Garde S 2011 Annu. Rev. Chem. Biomol. Eng. 2 147 |
| [5] | Santos L A, da C E F F, Freitas M P, and Ramalho T C 2014 J. Phys. Chem. A 118 5808 |
| [6] | Jiong S and Xu H 2021 Acta Polymer. Sin. 52 857 (in Chinese) |
| [7] | Milovanovic D, Honigmann A, Koike S, Göttfert F et al. 2015 Nat. Commun. 6 5984 |
| [8] | Baldwin R L and Rose G D 2016 Proc. Natl. Acad. Sci. USA 113 12462 |
| [9] | Durell S R and Ben-Naim A 2017 Biopolymers 107 e23020 |
| [10] | Gunasekara R W and Zhao Y 2017 Org. Lett. 19 4159 |
| [11] | Wong C K, Mason A F, Stenzel M H, and Thordarson P 2017 Nat. Commun. 8 1240 |
| [12] | Sun Q, Wang W, and Cui S 2021 Chem. Phys. 547 111200 |
| [13] | Lum K, Chandler D, and Weeks J D 1999 J. Phys. Chem. B 103 4570 |
| [14] | Chandler D 2005 Nature 437 640 |
| [15] | Hummer G, Garde S, Garcı́a A E, and Pratt L R 2000 Chem. Phys. 258 349 |
| [16] | Rajamani S, Truskett T M, and Garde S 2005 Proc. Natl. Acad. Sci. USA 102 9475 |
| [17] | Xue Y, Li X, Li H, and Zhang W 2014 Nat. Commun. 5 4348 |
| [18] | Cai W, Xu D, Qian L, Wei J, Xiao C, Qian L, Lu Z Y, and Cui S 2019 J. Am. Chem. Soc. 141 9500 |
| [19] | Li Y, Cheng J, Delparastan P, Wang H, Sigg S J, DeFrates K G, Cao Y, and Messersmith P B 2020 Nat. Commun. 11 3895 |
| [20] | Tian Y, Cao X, Li X et al. 2020 J. Am. Chem. Soc. 142 18687 |
| [21] | Xiao X, Liu C, Pei Y et al. 2020 J. Am. Chem. Soc. 142 3340 |
| [22] | Cai W, Xu D, Zhang F, Wei J, Lu S, Qian L, Lu Z Y, and Cui S 2022 Nano Res. 15 1517 |
| [23] | Guo Z, Hong H, Sun H, Zhang X, Wu C X, Li B, Cao Y, and Chen H 2021 Nanoscale 13 11262 |
| [24] | Lei H, Ma Q, Li W, Wen J, Ma H, Qin M, Wang W, and Cao Y 2021 Nat. Commun. 12 5082 |
| [25] | Zhang J, Wong S H D, Wu X, Lei H, Qin M, Shi P, Wang W, Bian L, and Cao Y 2021 Adv. Mater. 33 2105765 |
| [26] | Guo Z, Hong H, Yuan G, Qian H, Li B, Cao Y, Wang W, Wu C X, and Chen H 2020 Phys. Rev. Lett. 125 198101 |
| [27] | Xing H, Li Z D, Wang W B, Liu P R, Liu J K, Song Y, Wu Z L, Zhang W K, and Huang F H 2019 CCS Chem. 1 513 |
| [28] | Shi S C, Wang Z Y, Deng Y B, Tian F, Wu Q S, and Zheng P 2021 CCS Chem. 3 841 |
| [29] | Zhang J, Lei H, Qin M, Wang W, and Cao Y 2022 Supramolecular Mater. 1 100005 |
| [30] | Li I T S and Walker G C 2010 J. Am. Chem. Soc. 132 6530 |
| [31] | Li I T S and Walker G C 2011 Proc. Natl. Acad. Sci. USA 108 16527 |
| [32] | Li I T S and Walker G C 2012 Acc. Chem. Res. 45 2011 |
| [33] | Mondal J, Halverson D, Li I T S, Stirnemann G, Walker G C, and Berne B J 2015 Proc. Natl. Acad. Sci. USA 112 9270 |
| [34] | Di W, Gao X, Huang W et al. 2019 Phys. Rev. Lett. 122 047801 |
| [35] | Faghihnejad A and Zeng H 2012 Soft Matter 8 2746 |
| [36] | Cui X, Shi C, Xie L, Liu J, and Zeng H 2016 Langmuir 32 11236 |
| [37] | Parker J L, Claesson P M, and Attard P 1994 J. Phys. Chem. 98 8468 |
| [38] | Meyer E E, Rosenberg K J, and Israelachvili J 2006 Proc. Natl. Acad. Sci. USA 103 15739 |
| [39] | Xie L, Cui X, Gong L, Chen J, and Zeng H 2020 Langmuir 36 2985 |
| [40] | Pan Y, Huang S, Li F, Zhao X, and Wang W 2018 J. Mater. Chem. A 6 15057 |
| [41] | Xin-Wei W, Yong-Xin S, and Hao W 2012 Chin. Phys. Lett. 29 114702 |
| [42] | Ahmad D, van den Boogaert I, Miller J, Presswell R, and Jouhara H 2018 Energy Sources Part. A: Recovery Utilization Environ. Eff. 40 2686 |
| [43] | Cai Y, Li J, Yi L, Yan X, and Li J 2018 Appl. Surf. Sci. 450 102 |
| [44] | Salam A, Lucia L A, and Jameel H 2015 Cellulose 22 397 |
| [45] | Li-Xing L, Yuan D, and Yao W 2013 Chin. Phys. Lett. 30 108104 |
| [46] | Boban M, Golovin K, Tobelmann B, Gupte O, Mabry J M, and Tuteja A 2018 ACS Appl. Mater. & Interfaces 10 11406 |
| [47] | Chen J F, Xiao W J, Li D, Yang Y Y, and He Z H 2008 Chin. Phys. Lett. 25 747 |
| [48] | Wang Y and Gong X 2017 J. Mater. Chem. A 5 3759 |
| [49] | Hare E F, Shafrin E G, and Zisman W A 1954 J. Phys. Chem. 58 236 |
| [50] | Nishino T, Meguro M, Nakamae K, Matsushita M, and Ueda Y 1999 Langmuir 15 4321 |
| [51] | Gattás-Asfura K M and Stabler C L 2009 Biomacromolecules 10 3122 |
| [52] | Lansalot M, Davis T P, and Heuts J P A 2002 Macromolecules 35 7582 |
| [53] | Puts G, Venner V, Améduri B, and Crouse P 2018 Macromolecules 51 6724 |
| [54] | Köhn M and Breinbauer R 2004 Angew. Chem. Int. Ed. 43 3106 |
| [55] | Liu S and Edgar K J 2015 Biomacromolecules 16 2556 |
| [56] | Marko J F and Siggia E D 1995 Macromolecules 28 8759 |
| [57] | Bao Y, Luo Z, and Cui S 2020 Chem. Soc. Rev. 49 2799 |
| [58] | Lin J W P, Dudek L P, and Majumdar D 1987 J. Appl. Polym. Sci. 33 657 |
| [59] | Huang X, Zhou R, and Berne B J 2005 J. Phys. Chem. B 109 3546 |
| [60] | Wallqvist A, Gallicchio E, and Levy R M 2001 J. Phys. Chem. B 105 6745 |
| [61] | Huang D M and Chandler D 2002 J. Phys. Chem. B 106 2047 |
| [62] | Mittal J and Hummer G 2008 Proc. Natl. Acad. Sci. USA 105 20130 |
|
|
Viewed |
|
|
|
Full text
|
|
|
|
|
Abstract
|
|
|
|
|