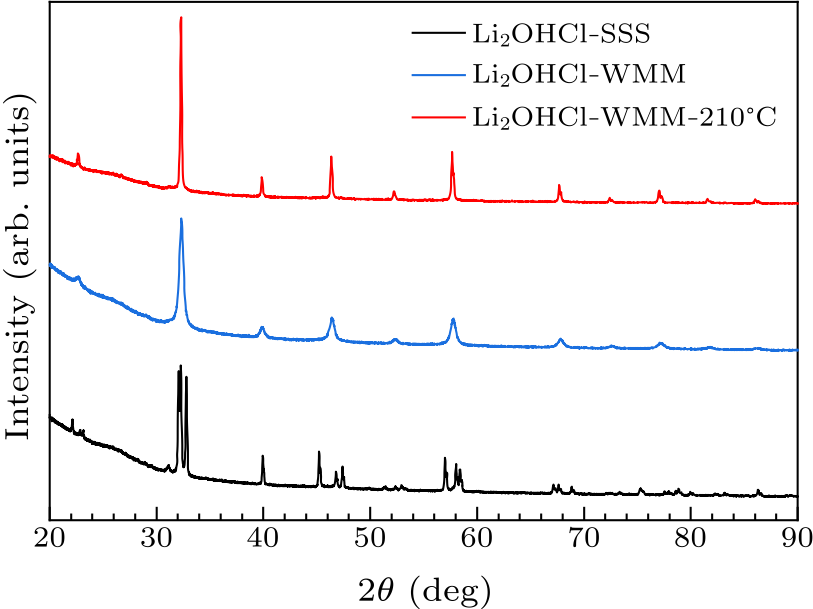


Fig. 1. PXRD patterns plot for Li$_{2}$OHCl-SSS, Li$_{2}$OHCl-WMM, and Li$_{2}$OHCl-WMM-210 ℃.

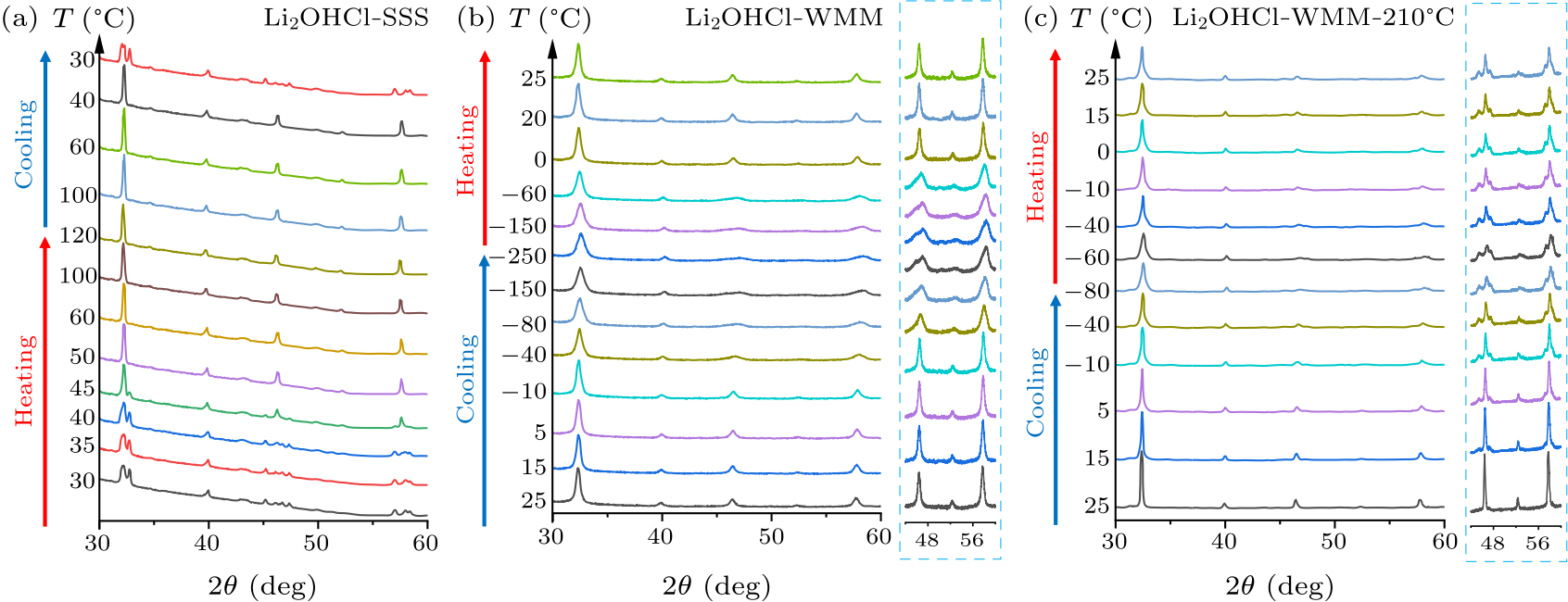
Fig. 2. PXRD patterns at temperatures from 30 to 120 ℃ for (a) Li$_{2}$OHCl-SSS, $-250$ to 25 ℃, (b) Li$_{2}$OHCl-WMM, $-100$ to 25 ℃, and (c) Li$_{2}$OHCl-WMM-210 ℃.

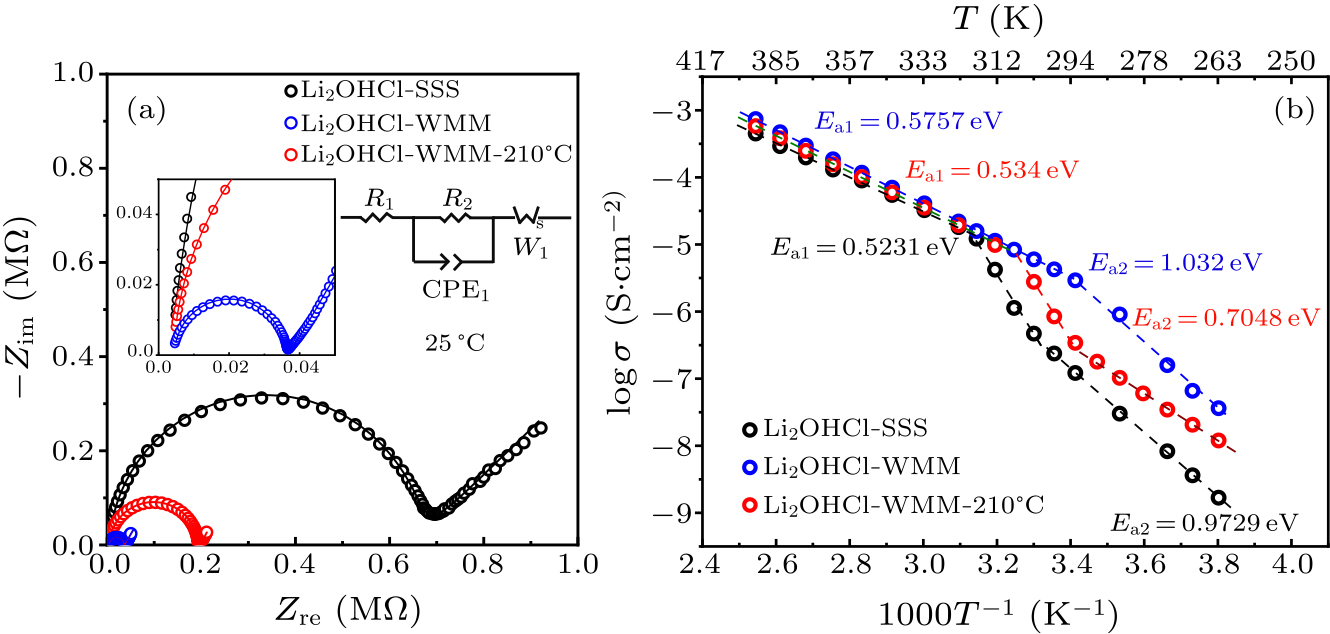
Fig. 3. (a) Typical Nyquist plots of Li$_{2}$OHCl-SSS (black), Li$_{2}$OHCl-WMM (blue), and Li$_{2}$OHCl-WMM-210 ℃ (red). (b) Arrhenius plots of temperature versus ionic conductivity for Li$_{2}$OHCl-SSS, Li$_{2}$OHCl-WMM and Li$_{2}$OHCl-WMM-210 ℃.

Fig. 4. SEM images for the Li$_{2}$OHCl synthesized by (a) solid-state sintering, (b) wet mechanical milling and 210 ℃ heating, and (c) wet mechanical milling.
| [1] | Zhang X, Wang S, Xue C, Xin C, Lin Y, Shen Y, Li L, and Nan C W 2019 Adv. Mater. 31 1806082 | Self‐Suppression of Lithium Dendrite in All‐Solid‐State Lithium Metal Batteries with Poly(vinylidene difluoride)‐Based Solid Electrolytes
| [2] | Sun C, Liu J, Gong Y, Wilkinson D P, and Zhang J 2017 Nano Energy 33 363 | Recent advances in all-solid-state rechargeable lithium batteries
| [3] | Kim S, Oguchi H, Toyama N et al. 2019 Nat. Commun. 10 1081 | A complex hydride lithium superionic conductor for high-energy-density all-solid-state lithium metal batteries
| [4] | Kim K M, Shin D O, and Lee Y G 2015 Electrochim. Acta 176 1364 | Effects of preparation conditions on the ionic conductivity of hydrothermally synthesized Li1+Al Ti2-(PO4)3 solid electrolytes
| [5] | Ohta H, Mizoguchi T, Aoki N, Yamamoto T, Sabarudin A, and Umemura T 2013 Appl. Phys. Lett. 102 089902 | Retraction: “Lithium-ion conducting La 2/3– x Li 3 x TiO 3 solid electrolyte thin films with stepped and terraced surfaces” [Appl. Phys. Lett. 100, 173107 (2012)]
| [6] | Han F, Zhu Y, He X, Mo Y, and Wang C 2016 Adv. Energy Mater. 6 1501590 | Electrochemical Stability of Li 10 GeP 2 S 12 and Li 7 La 3 Zr 2 O 12 Solid Electrolytes
| [7] | Hood Z D, Wang H, Samuthira P A, Keum J K, and Liang C 2016 J. Am. Chem. Soc. 138 1768 | Li 2 OHCl Crystalline Electrolyte for Stable Metallic Lithium Anodes
| [8] | Li Y, Zhou W, Xin S, Li S, Zhu J, Lu X, Cui Z, Jia Q, Zhou J, Zhao Y, and Goodenough J B 2016 Angew. Chem. Int. Ed. Engl. 55 9965 | Fluorine‐Doped Antiperovskite Electrolyte for All‐Solid‐State Lithium‐Ion Batteries
| [9] | Deng Z, Ou M, Wan J et al. 2020 Chem. Mater. 32 8827 | Local Structural Changes and Inductive Effects on Ion Conduction in Antiperovskite Solid Electrolytes
| [10] | Koedtruad A, Patino M A, Ichikawa N, Kan D, and Shimakawa Y 2020 J. Solid State Chem. 286 121263 | Crystal structures and ionic conductivity in Li2OHX (X = Cl, Br) antiperovskites
| [11] | Howard J, Hood Z D, and Holzwarth N A W 2017 Phys. Rev. Mater. 1 75406 | Fundamental aspects of the structural and electrolyte properties of from simulations and experiment
| [12] | Song A Y, Xiao Y, Turcheniuk K, Upadhya P, Ramanujapuram A, Benson J, Magasinski A, Olguin M, Meda L, Borodin O, and Yushin G 2018 Adv. Energy Mater. 8 1700971 | Protons Enhance Conductivities in Lithium Halide Hydroxide/Lithium Oxyhalide Solid Electrolytes by Forming Rotating Hydroxy Groups
| [13] | Song A Y, Turcheniuk K, Leisen J, Xiao Y, Meda L, Borodin O, and Yushin G 2020 Adv. Energy Mater. 10 1903480 | Understanding Li‐Ion Dynamics in Lithium Hydroxychloride (Li 2 OHCl) Solid State Electrolyte via Addressing the Role of Protons
| [14] | Georg S M J 2003 ChemPhysChem 4 343 | High Lithium Ionic Conductivity in the Lithium Halide Hydrates Li3-n(OHn)Cl (0.83≤n≤2) and Li3-n(OHn)Br (1≤n≤2) at Ambient Temperatures
| [15] | Yamamoto T, Shiba H, Mitsukuchi N, Sugumar M K, Motoyama M, and Iriyama Y 2020 Inorg. Chem. 59 11901 | Synthesis of the Metastable Cubic Phase of Li 2 OHCl by a Mechanochemical Method
| [16] | Gaffet E, Louison C, Harmelin M, and Faudot F 1991 Mater. Sci. Eng. A 34 1380 | Metastable phase transformations induced by ball-milling in the CuW system
| [17] | El-Eskandarany M S, Banyan M, and Al-Ajmi F 2018 RSC Adv. 8 32003 | Discovering a new MgH 2 metastable phase
| [18] | Hara K O, Yamasue E, Okumura H, and Ishihara K N 2009 J. Phys.: Conf. Ser. 144 012021 | Formation of metastable phases by high-energy ball milling in the Ti-O system