| Samples | Theoretical molar content of | Experimental molar content of |
|---|---|---|
| Li : Ni : Ti : Mo | Li : Ni : Ti : Mo | |
| Li$_{1.14}$Ni$_{0.57}$Ti$_{0.19}$Mo$_{0.10}$O$_{2 }(x=0$) | $1.14\!:\!0.57\!:\!0.19\!:\!0.10$ | $1.115\!:\!0.576\!:\!0.195\!:\!0.114$ |
| Li$_{1.14}$Ni$_{0.595}$Ti$_{0.165}$Mo$_{0.10}$O$_{1.95}$F$_{0.05 }(x=0.05$) | $1.14\!:\!0.595\!:\!0.165\!:\!0.10$ | $1.172\!:\!0.568\!:\!0.156\!:\!0.104$ |
| Li$_{1.14}$Ni$_{0.620}$Ti$_{0.140}$Mo$_{0.10}$O$_{1.90}$F$_{0.10 }(x=0.10$) | $1.14\!:\!0.620\!:\!0.140\!:\!0.10$ | $1.134\!:\!0.626\!:\!0.138\!:\!0.102$ |
| Li$_{1.14}$Ni$_{0.645}$Ti$_{0.115}$Mo$_{0.10}$O$_{1.85}$F$_{0.15 }(x=0.15$) | $1.14\!:\!0.645\!:\!0.115\!:\!0.10$ | $1.132\!:\!0.666\!:\!0.100\!:\!0.102$ |
| Li$_{1.14}$Ni$_{0.670}$Ti$_{0.090}$Mo$_{0.10}$O$_{1.80}$F$_{0.20 }(x=0.20$) | $1.14\!:\!0.670\!:\!0.090\!:\!0.10$ | $1.174\!:\!0.638\!:\!0.085\!:\!0.103$ |
| Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0,\, 0.05,\, 0.10,\, 0.15$, and 0.20) | $I$(003)$_{\rm L}$/$I$(104)$_{\rm L}$ |
|---|---|
| Li$_{1.14}$Ni$_{0.57}$Ti$_{0.19}$Mo$_{0.10}$O$_{2 }(x=0$) | 0.43 |
| Li$_{1.14}$Ni$_{0.595}$Ti$_{0.165}$Mo$_{0.10}$O$_{1.95}$F$_{0.05 }(x=0.05$) | 0.29 |
| Li$_{1.14}$Ni$_{0.620}$Ti$_{0.140}$Mo$_{0.10}$O$_{1.90}$F$_{0.10 }(x=0.10$) | 0.07 |
| Li$_{1.14}$Ni$_{0.645}$Ti$_{0.115}$Mo$_{0.10}$O$_{1.85}$F$_{0.15 }(x=0.15$) | 0.08 |
| Li$_{1.14}$Ni$_{0.670}$Ti$_{0.090}$Mo$_{0.10}$O$_{1.80}$F$_{0.20 }(x=0.20$) | 0.10 |
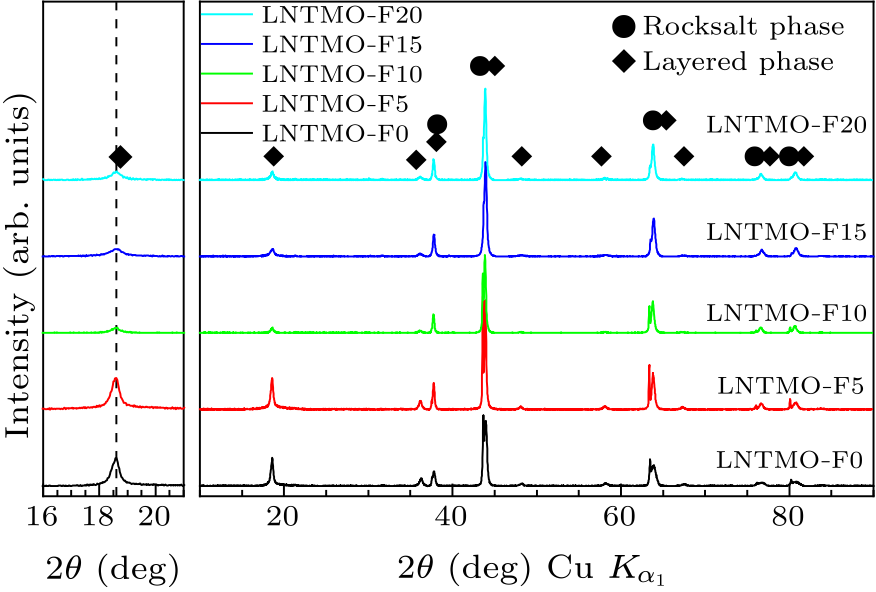
Fig. 1. x-ray diffraction patterns of Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0,\, 0.05,\, 0.10,\, 0.15$, and 0.20).
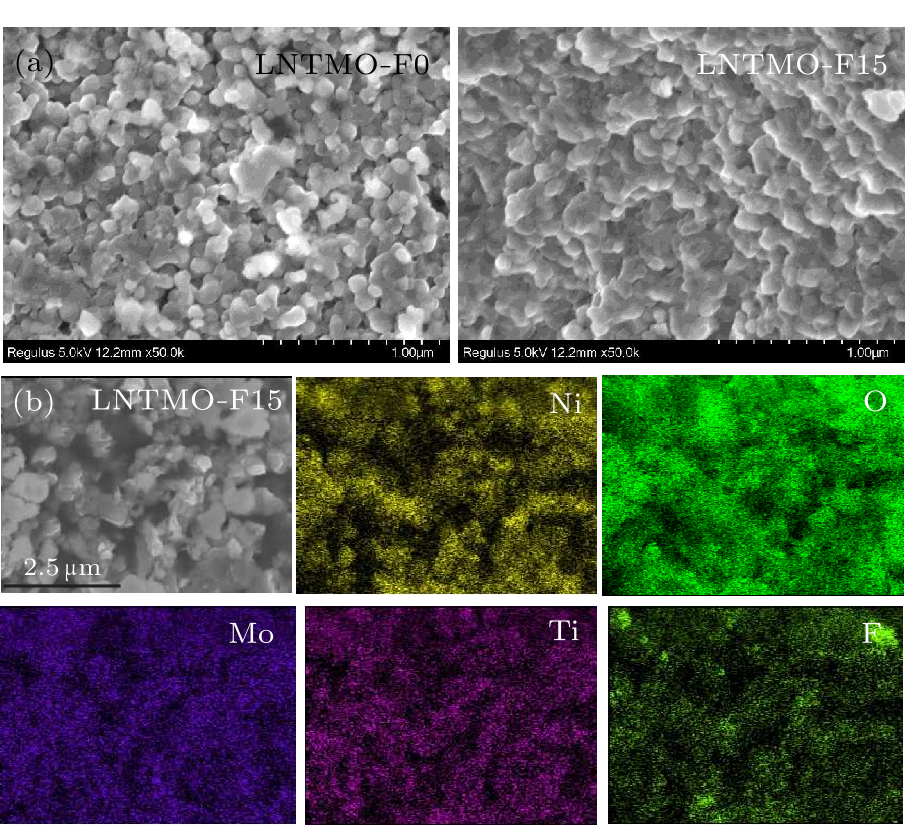
Fig. 2. (a) Morphological observation from SEM images of Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0,\, 0.15$). (b) EDS mapping of elemental distributions of Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0.15$).
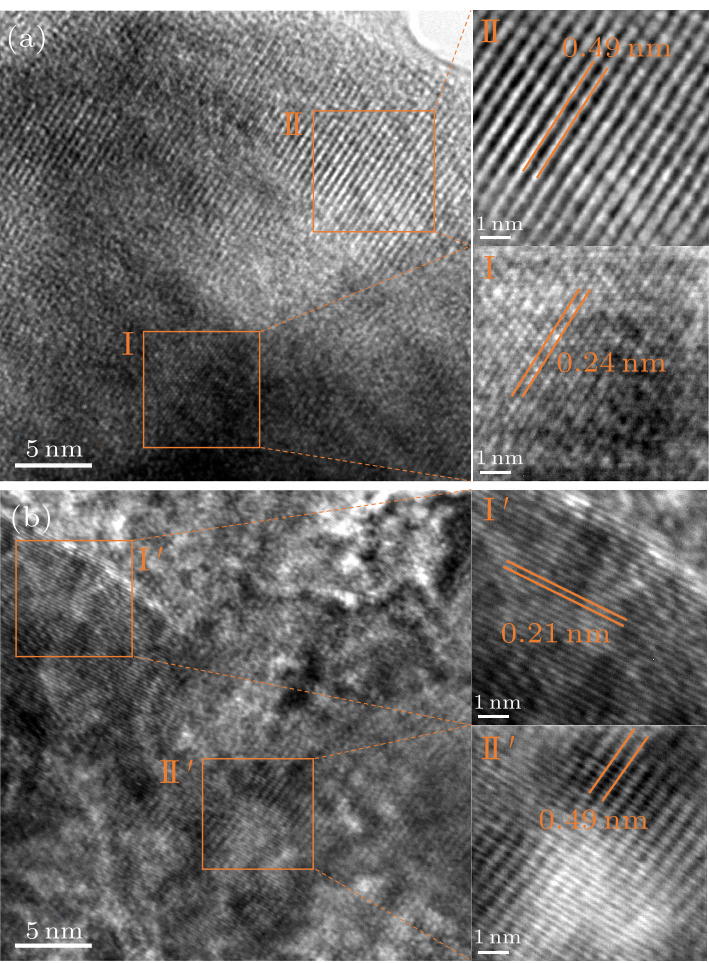
Fig. 3. HR-TEM images of (a) Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0$), (b) Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0.15$).
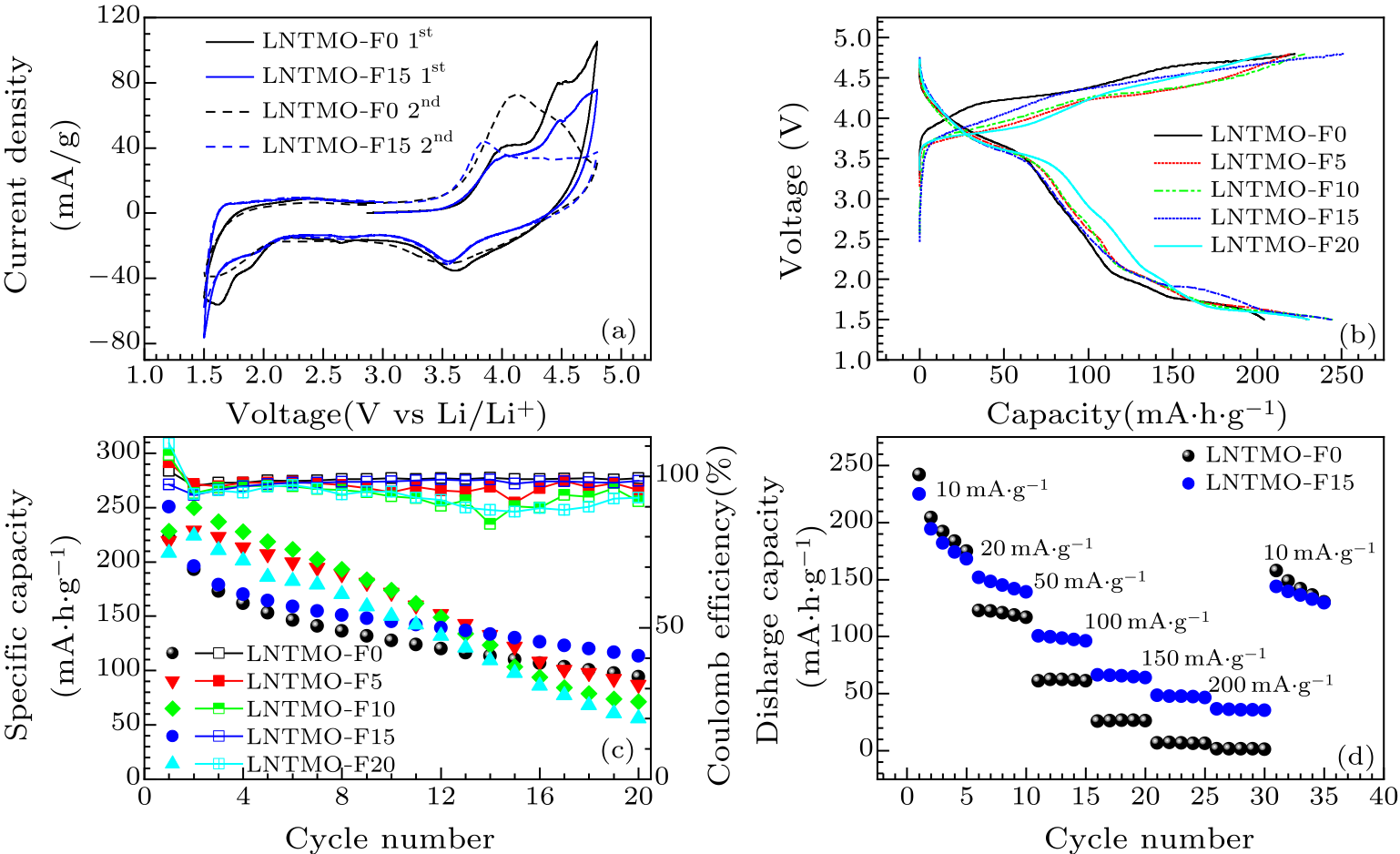
Fig. 4. (a) Cyclic voltammograms for the first two scans of Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0,\, 0.15$), with a scan rate of 0.1 mV/s. (b) Galvanostatic charge and discharge curves for the first cycle of Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0,\, 0.05,\, 0.10,\, 0.15$, and 0.20) within the voltage window of 1.5–4.8 V at 10 mA/g. (c) The capacity retention plots. (d) Rate capability of Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0,\, 0.15$).
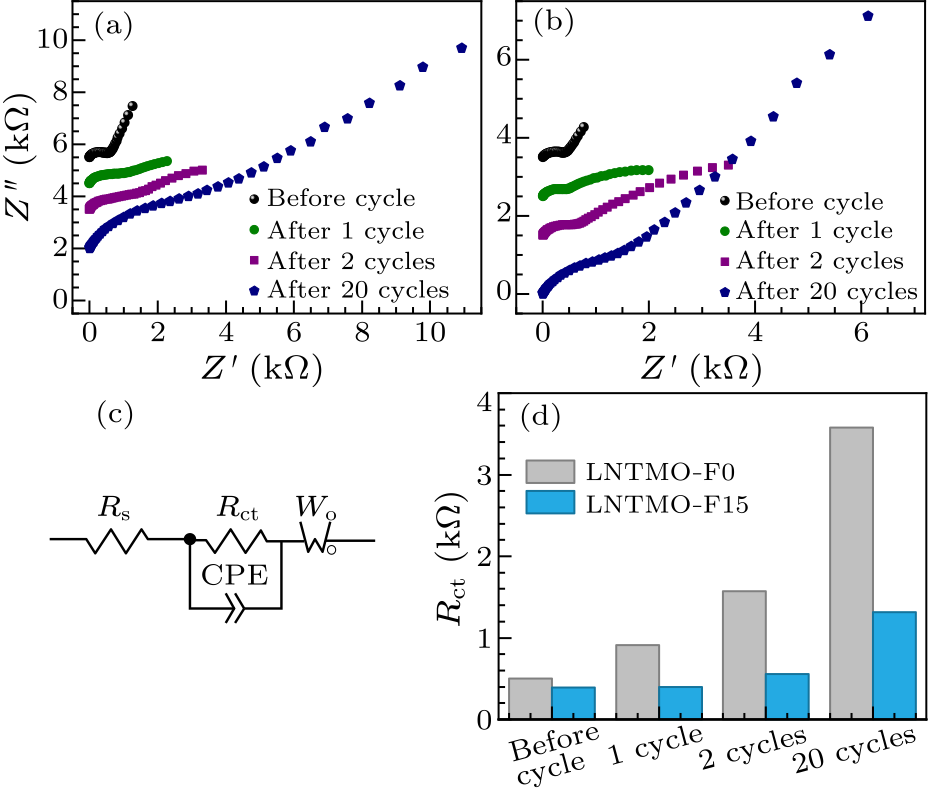
Fig. 5. Nyquist plots at different cycles of (a) Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0$), (b) Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0.15$). (c) Equivalent circuit model of Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0,\, 0.15$). The parameters $R_{\rm s}$ and $R_{\rm ct}$ refer to the ohm resistance and the charge transfer resistance, while $C_{\rm PE}$ and $W_{\rm o}$ correspond to the constant phase-angle element and the Warburg impedance, respectively. (d) Evaluated impedance parameters of $R_{\rm ct}$.
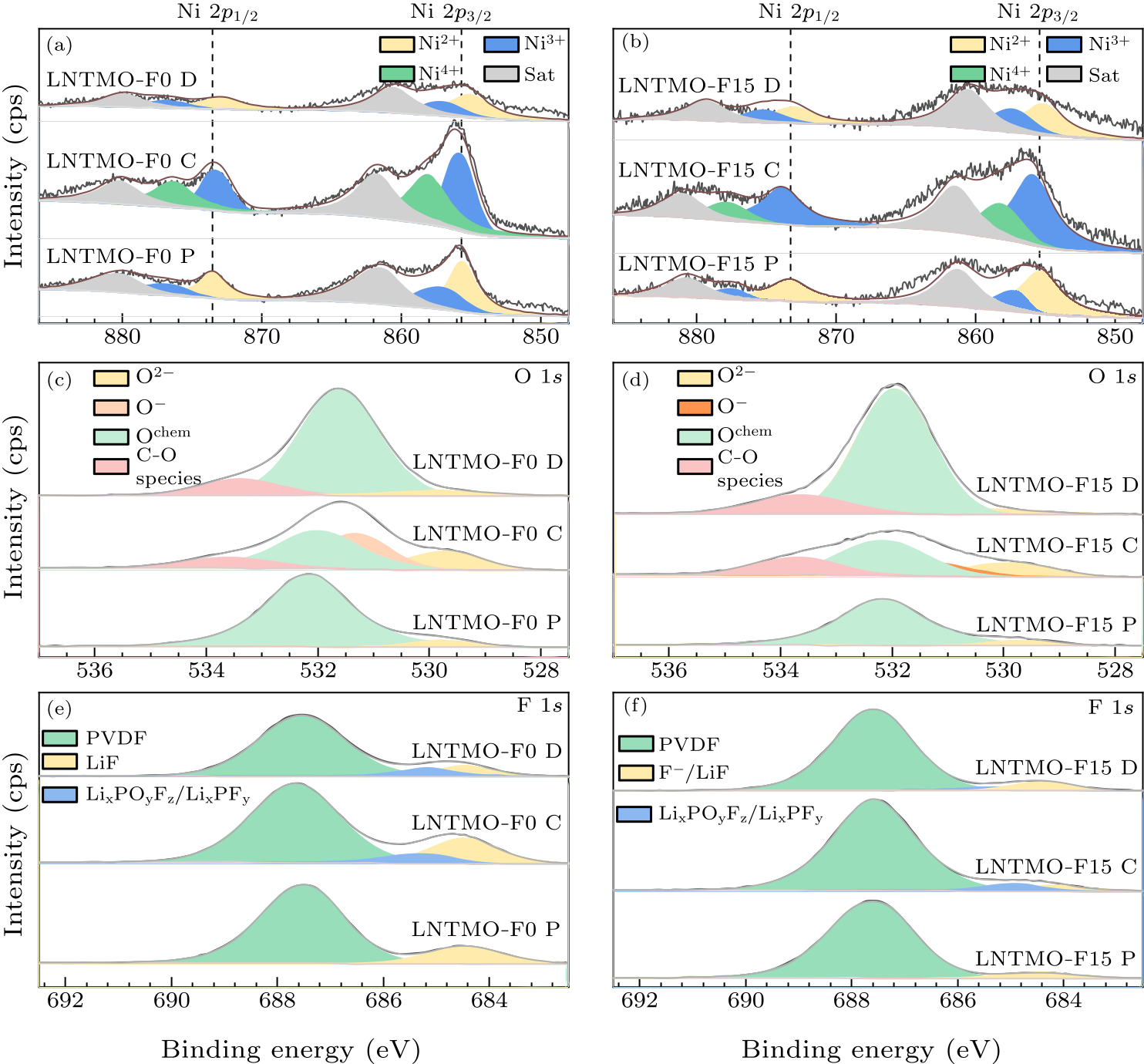
Fig. 6. x-ray photoelectron spectroscopy of the pristine, first charged and first discharged of Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2- x}$F$_{x}$ ($x=0,\, 0.15$) electrode: [(a), (b)] nickel $2p$, [(c), (d)] oxygen $1s$, [(e), (f)] fluorine $1s$.
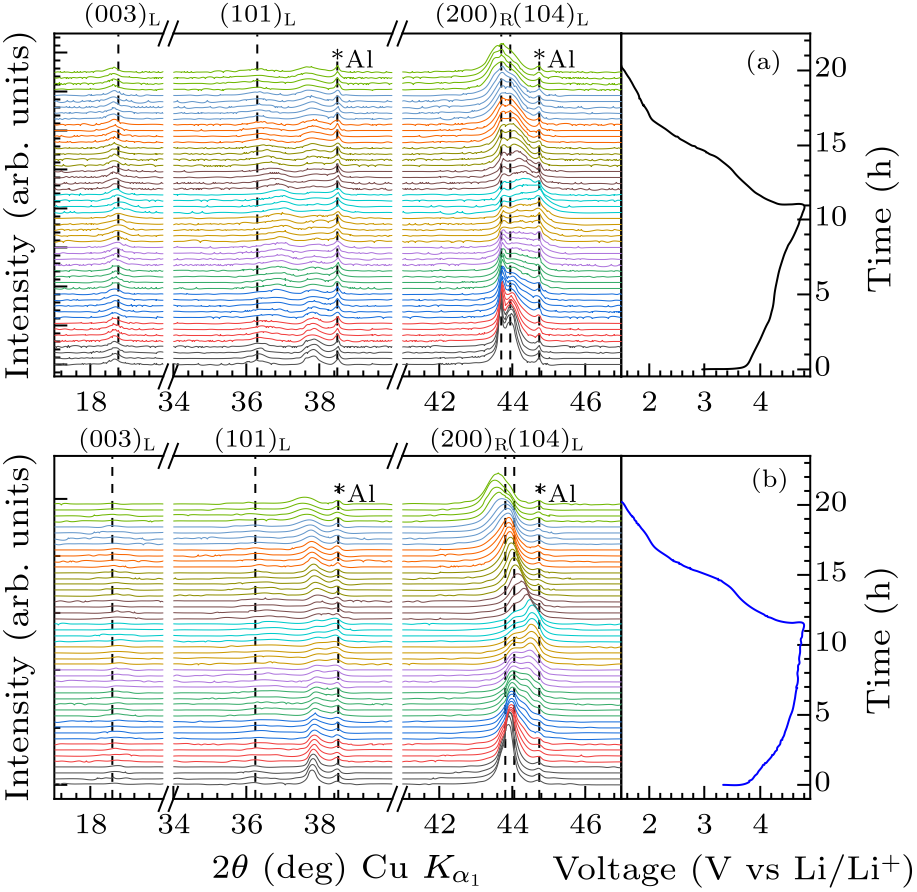
Fig. 7. In situ XRD patterns for the initial cycle of (a) Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0$), (b) Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0.15$) with the corresponding voltage profile.
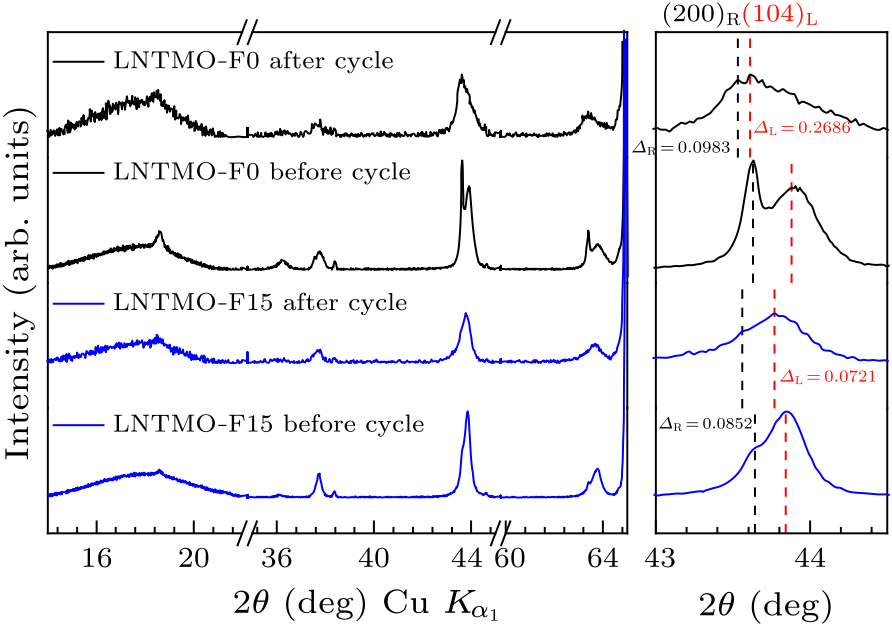
Fig. 8. Ex situ XRD patterns of Li$_{1.14}$Ni$_{0.57+0.5 x}$Ti$_{0.19-0.5 x}$Mo$_{0.10}$O$_{2-x}$F$_{x}$ ($x=0,\, 0.15$) before cycle and after 20 cycles.
| [1] | Yoshino A 2012 Angew. Chem. Int. Ed. Engl. 51 5798 | The Birth of the Lithium-Ion Battery
| [2] | Gupta A, Mullins C B, and Goodenough J B 2013 J. Power Sources 243 817 | Na 2 Ni 2 TeO 6 : Evaluation as a cathode for sodium battery
| [3] | Goodenough J B and Park K S 2013 J. Am. Chem. Soc. 135 1167 | The Li-Ion Rechargeable Battery: A Perspective
| [4] | Sato T et al. 2018 J. Mater. Chem. A 6 13943 | Metastable and nanosize cation-disordered rocksalt-type oxides: revisit of stoichiometric LiMnO 2 and NaMnO 2
| [5] | Manthiram A 2020 Nat. Commun. 11 1550 | A reflection on lithium-ion battery cathode chemistry
| [6] | Liu Q et al. 2018 Nat. Energy 3 936 | Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping
| [7] | Manthiram A et al. 2016 Adv. Energy Mater. 6 1501010 | Nickel-Rich and Lithium-Rich Layered Oxide Cathodes: Progress and Perspectives
| [8] | Whittingham M S 2004 Chem. Rev. 104 4271 | Lithium Batteries and Cathode Materials
| [9] | Masquelier C and Croguennec L 2013 Chem. Rev. 113 6552 | Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries
| [10] | Hy S et al. 2016 Energy & Environ. Sci. 9 1931 | Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries
| [11] | Lee J et al. 2015 Energy & Environ. Sci. 8 3255 | A new class of high capacity cation-disordered oxides for rechargeable lithium batteries: Li–Ni–Ti–Mo oxides
| [12] | Hamaguchi M et al. 2020 Electrochim. Acta 354 136630 | Suppression of O-redox reactions by multivalent Cr in Li-excess O4 (M, M’ = Cr, Mn, and Ti) cathodes with layered and cation-disordered rock-salt structures
| [13] | Zou Y et al. 2017 Adv. Sci. 4 1600262 | Multishelled Ni-Rich Li(Ni x Co y Mn z )O 2 Hollow Fibers with Low Cation Mixing as High-Performance Cathode Materials for Li-Ion Batteries
| [14] | Zhang X et al. 2010 J. Power Sources 195 1292 | Minimization of the cation mixing in Li1+x(NMC)1−xO2 as cathode material
| [15] | Lee J et al. 2014 Science 343 519 | Unlocking the Potential of Cation-Disordered Oxides for Rechargeable Lithium Batteries
| [16] | Clément R J, Lun Z, and Ceder G 2020 Energy & Environ. Sci. 13 345 | Cation-disordered rocksalt transition metal oxides and oxyfluorides for high energy lithium-ion cathodes
| [17] | Urban A, Lee J, and Ceder G 2014 Adv. Energy Mater. 4 1400478 | The Configurational Space of Rocksalt-Type Oxides for High-Capacity Lithium Battery Electrodes
| [18] | Lun Z et al. 2020 Chem 6 153 | Design Principles for High-Capacity Mn-Based Cation-Disordered Rocksalt Cathodes
| [19] | Zhao E et al. 2019 Energy Storage Mater. 16 354 | Structural and mechanistic revelations on high capacity cation-disordered Li-rich oxides for rechargeable Li-ion batteries
| [20] | Yabuuchi N 2019 Chem. Rec. 19 690 | Material Design Concept of Lithium‐Excess Electrode Materials with Rocksalt‐Related Structures for Rechargeable Non‐Aqueous Batteries
| [21] | Takeda N et al. 2017 J. Power Sources 367 122 | Reversible Li storage for nanosize cation/anion-disordered rocksalt-type oxyfluorides: LiMoO 2 – x LiF (0 ≤ x ≤ 2) binary system
| [22] | Yabuuchi N et al. 2016 Chem. Mater. 28 416 | Synthesis and Electrochemical Properties of Li 4 MoO 5 –NiO Binary System as Positive Electrode Materials for Rechargeable Lithium Batteries
| [23] | Yabuuchi N et al. 2015 Proc. Natl. Acad. Sci. USA 112 7650 | High-capacity electrode materials for rechargeable lithium batteries: Li 3 NbO 4 -based system with cation-disordered rocksalt structure
| [24] | Yabuuchi N et al. 2016 Nat. Commun. 7 13814 | Origin of stabilization and destabilization in solid-state redox reaction of oxide ions for lithium-ion batteries
| [25] | Hoshino S et al. 2017 ACS Energy Lett. 2 733 | Reversible Three-Electron Redox Reaction of Mo 3+ /Mo 6+ for Rechargeable Lithium Batteries
| [26] | Nakajima M and Yabuuchi N 2017 Chem. Mater. 29 6927 | Lithium-Excess Cation-Disordered Rocksalt-Type Oxide with Nanoscale Phase Segregation: Li 1.25 Nb 0.25 V 0.5 O 2
| [27] | Twu N et al. 2015 Nano Lett. 15 596 | Designing New Lithium-Excess Cathode Materials from Percolation Theory: Nanohighways in Li x Ni 2–4 x /3 Sb x /3 O 2
| [28] | Glazier S L et al. 2015 Chem. Mater. 27 7751 | Characterization of Disordered Li (1+ x ) Ti 2 x Fe (1–3 x ) O 2 as Positive Electrode Materials in Li-Ion Batteries Using Percolation Theory
| [29] | Kitchaev D A et al. 2018 Energy & Environ. Sci. 11 2159 | Design principles for high transition metal capacity in disordered rocksalt Li-ion cathodes
| [30] | Wang R et al. 2015 Electrochem. Commun. 60 70 | A disordered rock-salt Li-excess cathode material with high capacity and substantial oxygen redox activity: Li 1.25 Nb 0.25 Mn 0.5 O 2
| [31] | Song B et al. 2013 J. Mater. Chem. A 1 9954 | Graphene-based surface modification on layered Li-rich cathode for high-performance Li-ion batteries
| [32] | Yang M et al. 2012 J. Mater. Chem. 22 6200 | Cation disordered rock salt phase Li2CoTiO4 as a potential cathode material for Li-ion batteries
| [33] | Clément R J et al. 2018 Chem. Mater. 30 6945 | Short-Range Order and Unusual Modes of Nickel Redox in a Fluorine-Substituted Disordered Rocksalt Oxide Lithium-Ion Cathode
| [34] | Lee J et al. 2017 Nat. Commun. 8 981 | Mitigating oxygen loss to improve the cycling performance of high capacity cation-disordered cathode materials
| [35] | Crafton M J et al. 2020 Adv. Energy Mater. 10 2001500 | Anion Reactivity in Cation‐Disordered Rocksalt Cathode Materials: The Influence of Fluorine Substitution
| [36] | Wang Y et al. 2018 J. Phys. Chem. C 122 27836 | Suppressing Mn Reduction of Li-Rich Mn-Based Cathodes by F-Doping for Advanced Lithium-Ion Batteries
| [37] | Ahn J, Chen D, and Chen G 2020 Adv. Energy Mater. 10 2001671 | A Fluorination Method for Improving Cation‐Disordered Rocksalt Cathode Performance
| [38] | Zhou K et al. 2020 Energy Storage Mater. 32 234 | Fluorination effect for stabilizing cationic and anionic redox activities in cation-disordered cathode materials
| [39] | Lee J et al. 2018 Nature 556 185 | Reversible Mn2+/Mn4+ double redox in lithium-excess cathode materials
| [40] | Xu J et al. 2020 Solid State Ionics 345 115172 | High capacity Li/Ni rich Ni-Ti-Mo oxide cathode for Li-ion batteries
| [41] | Huang Y et al. 2020 Solid State Ionics 351 115341 | A new model on cation distribution in cation-disordered Li1+xTM1−xO2 cathodes
| [42] | Shaju K M, Rao G V S, and Chowdari B V R 2002 Electrochim. Acta 48 145 | Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries
| [43] | Papp J K et al. 2021 Electrochim. Acta 368 137505 | A comparison of high voltage outgassing of LiCoO2, LiNiO2, and Li2MnO3 layered Li-ion cathode materials
| [44] | Levi E et al. 1999 Solid State Ionics 126 97 | Electrochemical and in-situ XRD characterization of LiNiO2 and LiCo0.2Ni0.8O2 electrodes for rechargeable lithium cells
| [45] | Shannon R D 1976 Acta Cryst. A 32 751 | Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides
| [46] | Wang X et al. 2017 J. Power Sources 359 270 | Attainable high capacity in Li-excess Li-Ni-Ru-O rock-salt cathode for lithium ion battery
| [47] | Babu D B et al. 2019 J. Power Sources 436 226870 | Synthesis and investigation of electrochemical performance of mixed valent Li4FeMoO6 as positive electrode material in rechargeable lithium ion batteries
| [48] | Yu Z et al. 2019 ACS Appl. Mater. & Interfaces 11 35777 | Synthesis and Redox Mechanism of Cation-Disordered, Rock-Salt Cathode-Material Li–Ni–Ti–Nb–O Compounds for a Li-Ion Battery
| [49] | Zhang L et al. 2008 J. Power Sources 185 534 | Synthesis and electrochemistry of cubic rocksalt Li–Ni–Ti–O compounds in the phase diagram of LiNiO2–LiTiO2–Li[Li1/3Ti2/3]O2
| [50] | Zheng S et al. 2019 J. Alloys Compd. 773 1 | Electrochemistry and redox characterization of rock-salt-type lithium metal oxides Li1+z/3Ni1/2-z/2Ti1/2+z/6O2 for Li-ion batteries
| [51] | Shen C H et al. 2014 ACS Appl. Mater. & Interfaces 6 5516 | Facile Synthesis of The Li-Rich Layered Oxide Li 1.23 Ni 0.09 Co 0.12 Mn 0.56 O 2 with Superior Lithium Storage Performance and New Insights into Structural Transformation of the Layered Oxide Material during Charge–Discharge Cycle: In Situ XRD Characterization
| [52] | Qiao Y Q et al. 2011 J. Phys. Chem. C 115 25508 | Self-Assembled Synthesis of Hierarchical Waferlike Porous Li–V–O Composites as Cathode Materials for Lithium Ion Batteries
| [53] | Wang Z et al. 2019 Ceram. Int. 45 20016 | Surface modification of Li rich Li1.2Mn0.54Ni0.13Co0.13O2 cathode particles
| [54] | Assat G et al. 2016 J. Electrochem. Soc. 163 A2965 | Editors' Choice—Practical Assessment of Anionic Redox in Li-Rich Layered Oxide Cathodes: A Mixed Blessing for High Energy Li-Ion Batteries
| [55] | Naylor A J et al. 2020 ACS Appl. Energy Mater. 3 5937 | Stabilization of Li-Rich Disordered Rocksalt Oxyfluoride Cathodes by Particle Surface Modification
| [56] | Wei X et al. 2013 Electrochim. Acta 107 549 | Electrochemical performance of high-capacity nanostructured Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium ion battery by hydrothermal method
| [57] | Yang M et al. 2019 ACS Appl. Mater. & Interfaces 11 44144 | Cation-Disordered Lithium-Excess Li–Fe–Ti Oxide Cathode Materials for Enhanced Li-Ion Storage
| [58] | Xie Q et al. 2019 Chem. Mater. 31 8886 | Insights into Boron-Based Polyanion-Tuned High-Nickel Cathodes for High-Energy-Density Lithium-Ion Batteries
| [59] | Schulz N et al. 2018 J. Electrochem. Soc. 165 A833 | XPS-Surface Analysis of SEI Layers on Li-Ion Cathodes: Part II. SEI-Composition and Formation inside Composite Electrodes
| [60] | Andersson D P A A M et al. 2002 J. Electrochem. Soc. 149 A1358 | Surface Characterization of Electrodes from High Power Lithium-Ion Batteries
| [61] | Zheng S et al. 2020 ACS Appl. Mater. & Interfaces 12 40347 | Mn 4+ -Substituted Li-Rich Li 1.2 Mn 0.4 3+ Mn x 4+ Ti 0.4– x O 2 Materials with High Energy Density
| [62] | Li L et al. 2021 Adv. Funct. Mater. 31 2101888 | Fluorination‐Enhanced Surface Stability of Cation‐Disordered Rocksalt Cathodes for Li‐Ion Batteries
| [63] | Meng F et al. 2019 Ionics 25 1967 | Magnesium-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode with high rate capability and improved cyclic stability
| [64] | Huang B et al. 2014 J. Power Sources 252 200 | A comprehensive study on electrochemical performance of Mn-surface-modified LiNi0.8Co0.15Al0.05O2 synthesized by an in situ oxidizing-coating method