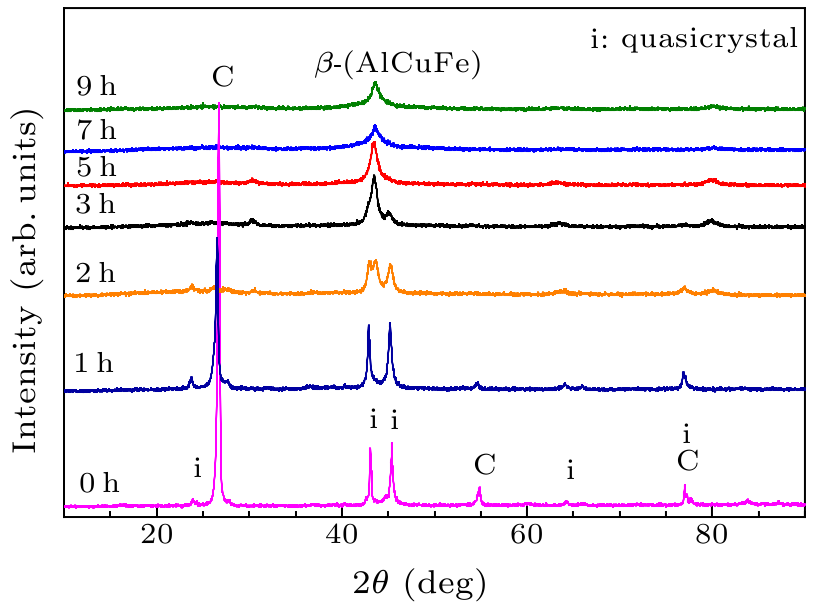
Fig. 1. X-ray diffraction spectra of mixed powders after high energy vibration ball milling at different times.
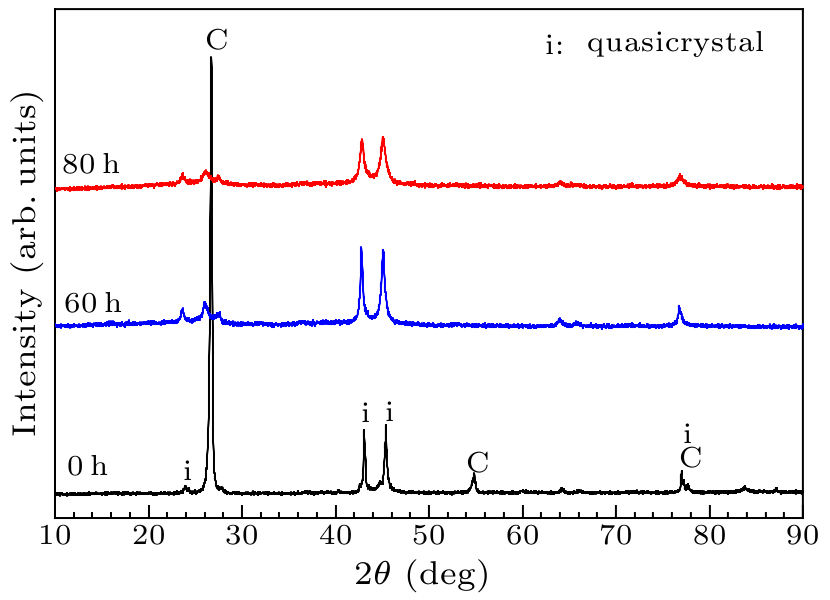
Fig. 2. XRD of planetary milled 550 rpm powders for different milling times.
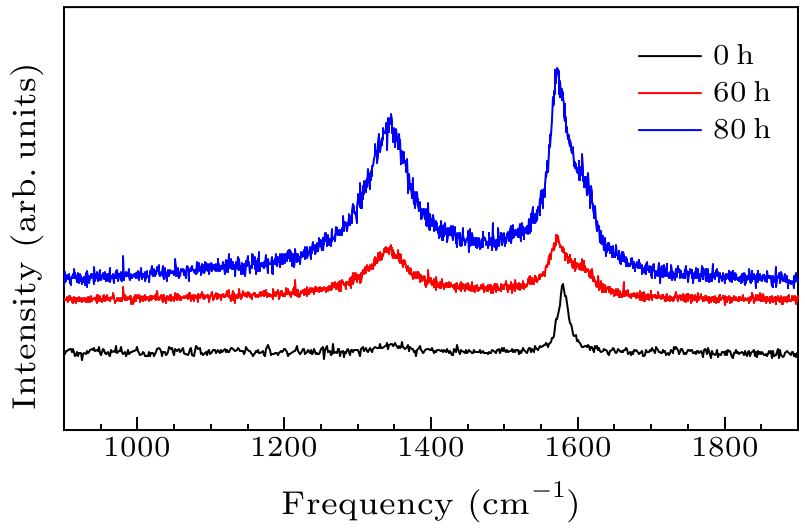
Fig. 3. Raman spectra of graphite milled by planetary ball at 550 rpm at different times.
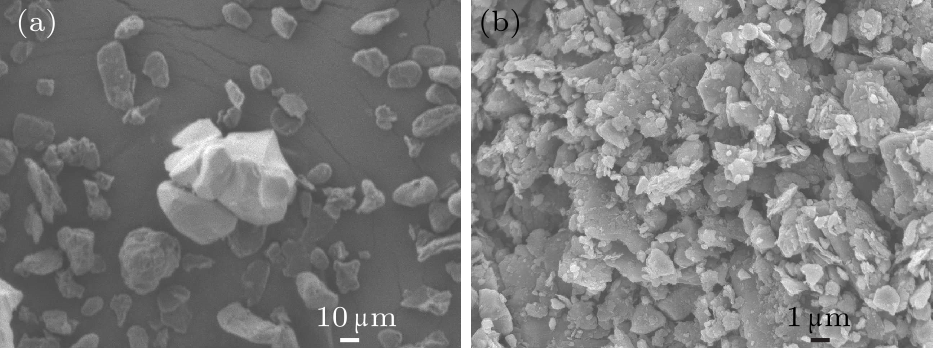
Fig. 4. (a) SEM image of the initial Al–Cu–Fe QCs and graphite mixed powders. (b) SEM image of graphite/AlCuFe QC composites after planetary ball milling for 80 h.
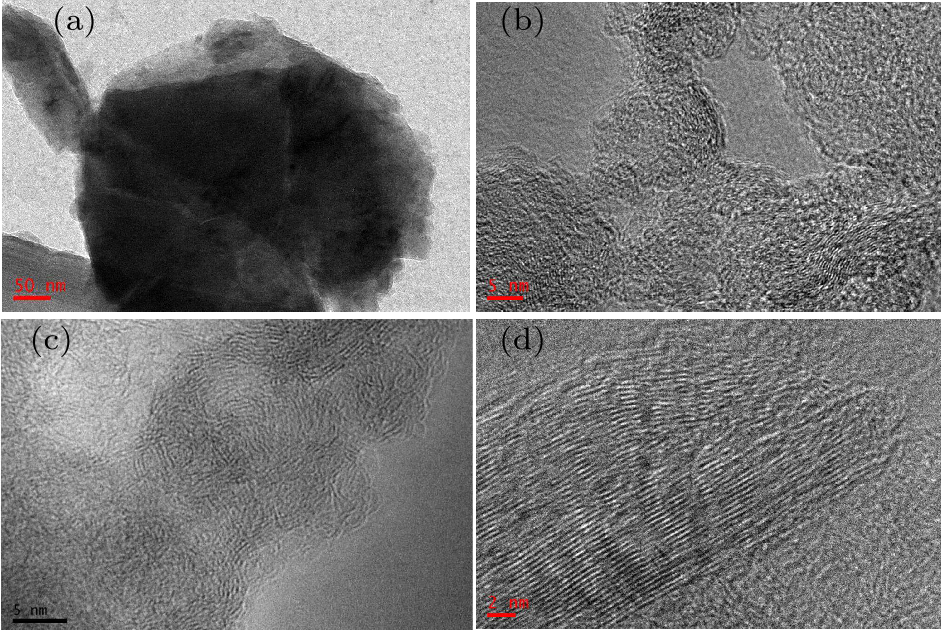
Fig. 5. [(a), (b)] TEM images of graphite/AlCuFe QC composites with different magnifications after planetary ball milling for 80 h. [(c), (d)] HRTEM images of graphite particles with different magnifications.
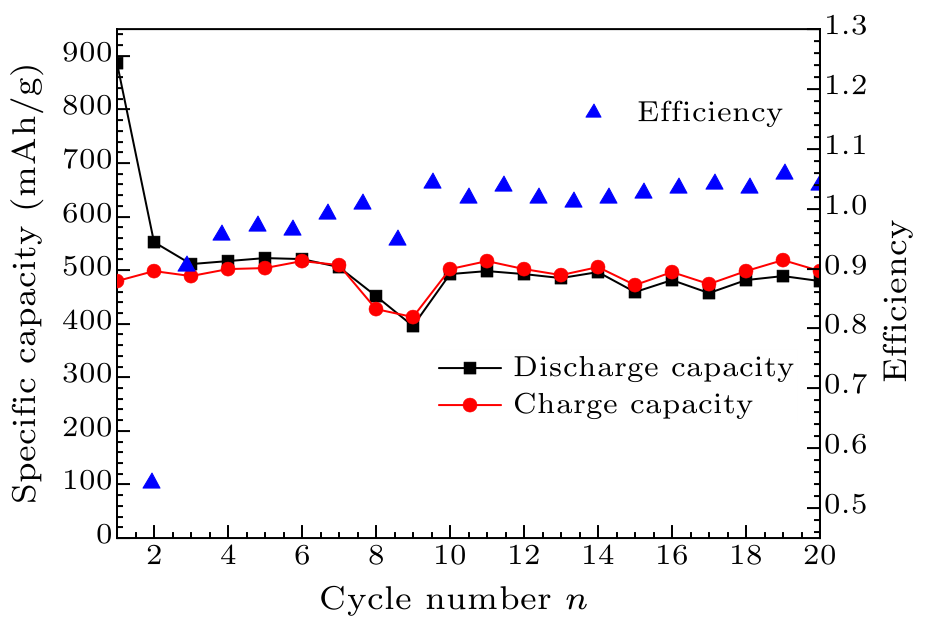
Fig. 6. Cyclic performance curve of graphite/AlCuFe QC composites.
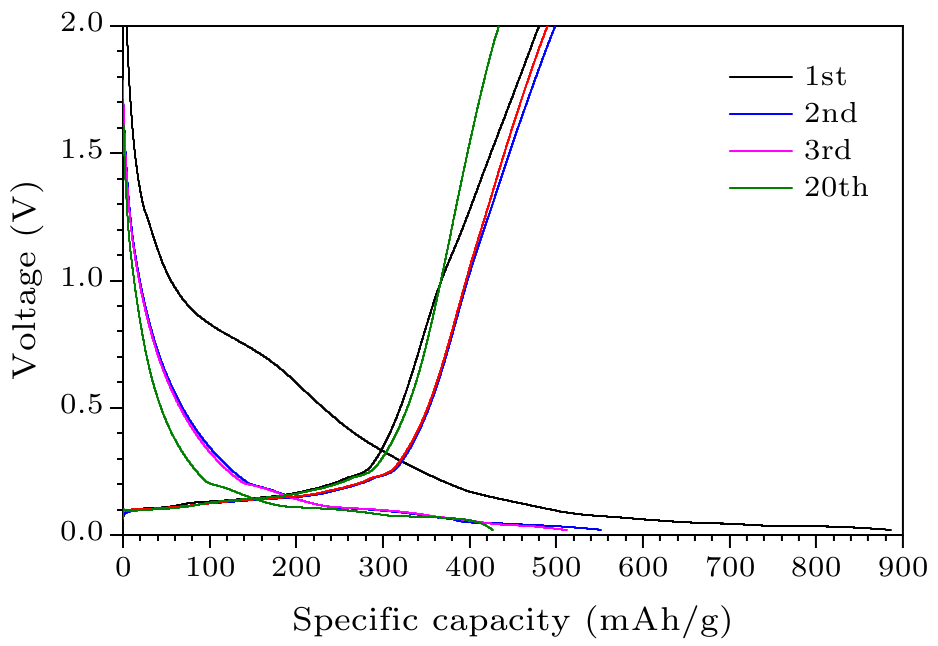
Fig. 7. The voltage between the graphite/AlCuFe QC anode and the Li/Li$^+$ electrode versus the specific capacity of graphite/AlCuFe QC composites from the first cycle to the 20th cycle.
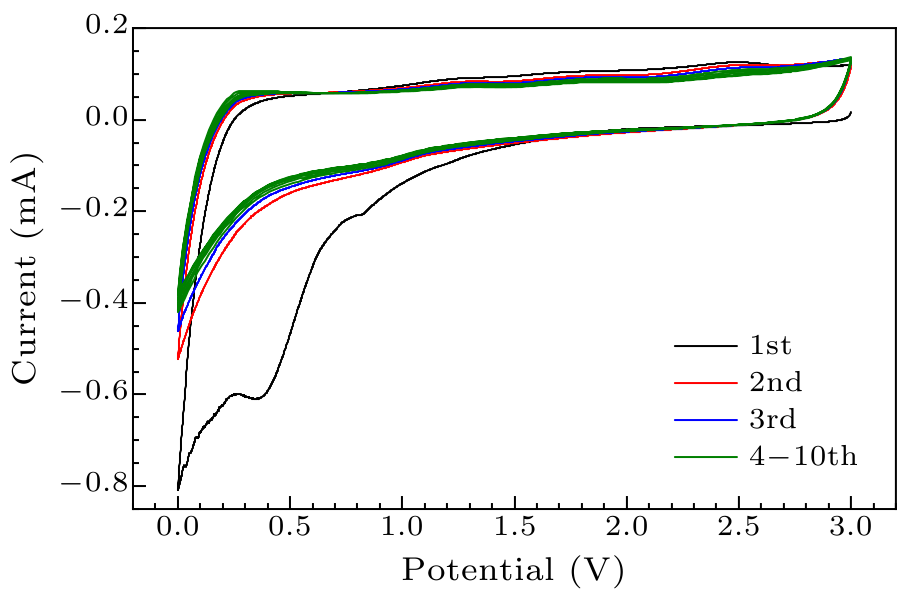
Fig. 8. Cyclic voltammetric curves of graphite/AlCuFe QC composites.
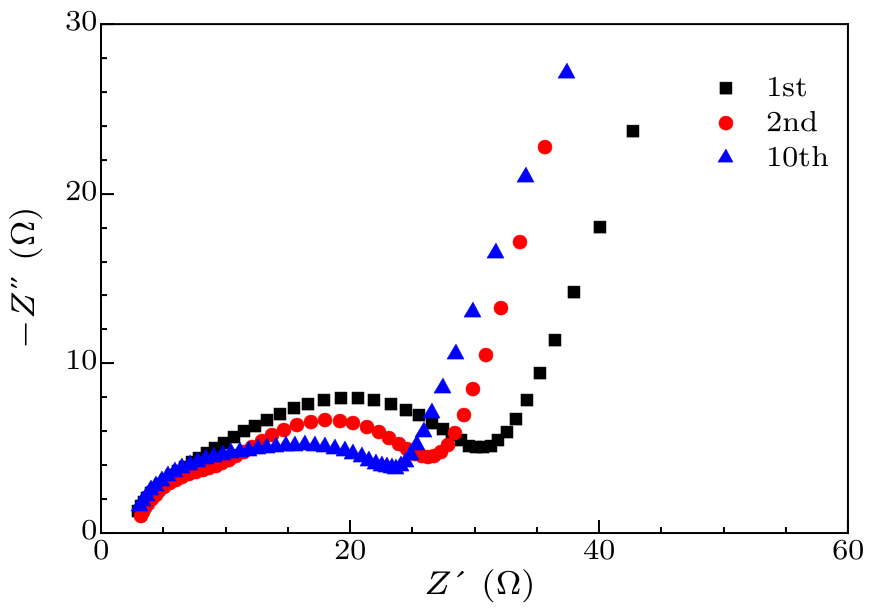
Fig. 9. Impedance spectra of graphite/AlCuFe QC composite anodes at different states.
| [1] | Key B, Morcrette M, Tarascon J M et al 2011 J. Am. Chem. Soc. 133 503 | Pair Distribution Function Analysis and Solid State NMR Studies of Silicon Electrodes for Lithium Ion Batteries: Understanding the (De)lithiation Mechanisms
| [2] | Hui W and Yi C 2012 Nano Today 7 414 | Designing nanostructured Si anodes for high energy lithium ion batteries
| [3] | Zhang W 2011 J. Power Sources 196 13 | A review of the electrochemical performance of alloy anodes for lithium-ion batteries
| [4] | Liu X H, Zhong L, Huang S et al 2012 ACS Nano 6 1522 | Size-Dependent Fracture of Silicon Nanoparticles During Lithiation
| [5] | Lei X, Wang C, Yi Z et al 2007 J. Alloys Compd. 429 311 | Effects of particle size on the electrochemical properties of aluminum powders as anode materials for lithium ion batteries
| [6] | Honda H, Sakaguchi H, Fukuda Y et al 2003 Mater. Res. Bull. 38 647 | Anode behaviors of aluminum antimony synthesized by mechanical alloying for lithium secondary battery
| [7] | Rouxel D and Pigeat P 2006 Prog. Surf. Sci. 81 488 | Surface oxidation and thin film preparation of AlCuFe quasicrystals
| [8] | Jiang X Y, Liu Q S and Zhang L 2011 Rare Met. 30 (Suppl. 1) 63 | Electrochemical hydrogen storage property of NiTi alloys with different Ti content prepared by mechanical alloying
| [9] | Luo X L, Grant D M and Walker G S 2015 J. Alloys Compd. 645 S23 | Hydrogen storage properties for Mg–Zn–Y quasicrystal and ternary alloys
| [10] | Lan X, Wang H, Sun Z et al 2019 J. Alloys Compd. 805 942 | Al–Cu–Fe quasicrystals as the anode for lithium ion batteries
| [11] | Patiño-Carachure C, Flores-Chan J E, Gil A F et al 2017 J. Alloys Compd. 694 46 | Synthesis of onion-like carbon-reinforced AlCuFe quasicrystals by high-energy ball milling
| [12] | Chan C K, Peng H, Liu G et al 2008 Nat. Nanotechnol. 3 31 | High-performance lithium battery anodes using silicon nanowires
| [13] | Fuchsbichler B, Stangl C, Kren H et al 2011 J. Power Sources 196 2889 | High capacity graphite–silicon composite anode material for lithium-ion batteries