| CONDENSED MATTER: STRUCTURE, MECHANICAL AND THERMAL PROPERTIES |
 |
|

|
|
|
Lithium Ion Batteries Operated at $-100\,^{\circ}\!$C |
| Jianli Gai1, Jirong Yang1, Wei Yang1*, Quan Li2, Xiaodong Wu1,4,5, and Hong Li1,2,3* |
1Tianmu Lake Institute of Advanced Energy Storage Technologies Co., Ltd., Liyang 213300, China
2Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China
3Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
4i-Lab, Suzhou Institute of Nano-Tech and Nano-Bionics (SINANO), Chinese Academy of Sciences, Suzhou 215123, China
5School of Nano-Tech and Nano-Bionics, University of Science and Technology of China, Hefei 230026, China
|
|
| Cite this article: |
|
Jianli Gai, Jirong Yang, Wei Yang et al 2023 Chin. Phys. Lett. 40 086101 |
|
|
|
|
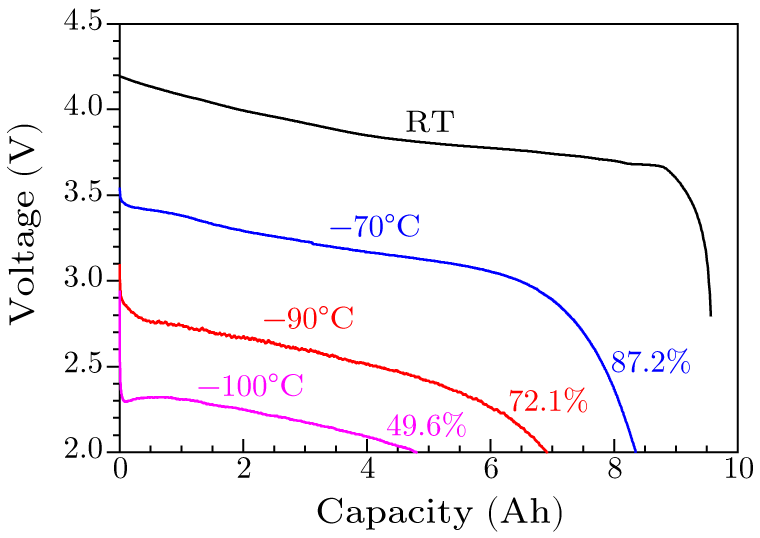
Abstract Enabling lithium-ion batteries (LIBs) to operate in a wider temperature range, e.g., as low or high as possible or capable of both, is an urgent need and shared goal. Here we report, for the first time, a low-temperature electrolyte consisting of traditional ethylene carbonate, methyl acetate, butyronitrile solvents, and 1 M LiPF$_{6}$ salt, attributed to its very low freezing point ($T_{\rm f} = -126.3\,^{\circ}\!$C) and high ion conductivity at extremely low temperatures (0.21 mS/cm at $-100\,^{\circ}\!$C), successfully extends the service temperature of a practical 9.6 Ah LIB down to $-100\,^{\circ}\!$C (49.6% capacity retention compared to that at room temperature), which is the lowest temperature reported for practical cells so far as we know, and is lower than the lowest natural temperature ($-89.2\,^{\circ}\!$C) recorded on earth. Meanwhile, the high-temperature performance of lithium-ion batteries is not affected. The capacity retention is 88.2% and 83.4% after 800 cycles at 25$\,^{\circ}\!$C and 45$\,^{\circ}\!$C, respectively. The progress also makes LIB a proper power supplier for space vehicles in astronautic explorations.
|
|
Received: 10 May 2023
Express Letter
Published: 04 July 2023
|
|
| PACS: |
61.20.Qg
|
(Structure of associated liquids: electrolytes, molten salts, etc.)
|
| |
66.10.-x
|
(Diffusion and ionic conduction in liquids)
|
| |
82.47.Aa
|
(Lithium-ion batteries)
|
| |
88.05.Gh
|
(Energy conservation; electricity demand reduction)
|
|
|
|
|
|
| [1] | Lin R J and Ren J Z 2020 Renewable Energy Sustain. Dev. 6 3 |
| [2] | Tarascon J M and Armand M 2001 Nature 414 359 |
| [3] | Plichta E J, Hendrickson M, Thompson R, Au G, Behl W K, Smart M C, Ratnakumar B V, and Surampudi S 2001 J. Power Sources 94 160 |
| [4] | Rodrigues M T F, Babu G, Gullapalli H, Kalaga K, Sayed F N, Kato K, Joyner J, and Ajayan P M 2017 Nat. Energy 2 17108 |
| [5] | Chen M Z, Zhang Y Y, Xing G C, Chou S L, and Tang Y X 2021 Energy & Environ. Sci. 14 3323 |
| [6] | Belgibayeva A, Rakhmetova A, Rakhatkyzy M et al. 2023 J. Power Sources 557 232550 |
| [7] | Cho Y G, Kim Y S, Sung D G, Seo M S, and Song H K 2014 Energy & Environ. Sci. 7 1737 |
| [8] | Gering K L 2006 ECS Trans. 1 119 |
| [9] | Zhang S S, Xu K, and Jow T R 2003 J. Power Sources 115 137 |
| [10] | Zhang W, Xia H, Zhu Z et al. 2021 CCS Chem. 3 1245 |
| [11] | Jones J P, Smart M C, Krause F C, West W C, and Brandon E J 2022 Joule 6 923 |
| [12] | Zhang S S, Xu K, and Jow T R 2002 Electrochem. Commun. 4 928 |
| [13] | Rustomji C S, Yang Y, Kim T K, Mac J, Kim Y J, Caldwell E, Chung H, and Meng Y S 2017 Science 356 eaal4263 |
| [14] | Dong X L, Guo Z W, Guo Z Y, Wang Y G, and Xia Y Y 2018 Joule 2 902 |
| [15] | Fan X L, Ji X, Chen L et al. 2019 Nat. Energy 4 882 |
| [16] | Nian Q, Wang J, Liu S, Sun T, Zheng S, Zhang Y, Tao Z, and Chen J 2019 Angew. Chem. Int. Ed. 58 16994 |
| [17] | Yang Y, Yin Y J, Davies D M et al. 2020 Energy & Environ. Sci. 13 2209 |
| [18] | Holoubek J, Liu H, Wu Z et al. 2021 Nat. Energy 6 303 |
| [19] | Li Q, Liu G, Cheng H, Sun Q, Zhang J, and Ming J 2021 Chem. - Eur. J. 27 15842 |
| [20] | Chae Y, Lim C, Jeon J, Kim M, Lee K K, Kwak K, and Cho M 2022 J. Phys. Chem. Lett. 13 7881 |
| [21] | Chen Z, Wang K, Pei P, Zuo Y, Wei M, Wang H, Zhang P, and Shang N 2023 Nano Res. 16 2311 |
| [22] | Huang J H, Dong X L, Wang N, and Wang Y G 2022 Curr. Opin. Electrochem. 33 100949 |
| [23] | Yoo D J, Liu Q, Cohen O, Kim M, Persson K A, and Zhang Z 2022 ACS Appl. Mater. & Interfaces 14 11910 |
| [24] | Liu J P, Yuan B T, He N D et al. 2023 Energy & Environ. Sci. 16 1024 |
| [25] | Xu J J, Zhang J X, Pollard T P et al. 2023 Nature 614 694 |
| [26] | Yang C Y, Xia J L, Cui C Y et al. 2023 Nat. Sustain. 6 325 |
| [27] | Yang Y, Fang Z, Yin Y, Cao Y, Wang Y, Dong X, and Xia Y 2022 Angew. Chem. Int. Ed. 61 e202213688 |
| [28] | Qin M S, Liu M C, Zeng Z Q, Wu Q, Wu Y K, Zhang H, Lei S, Cheng S J, and Xie J 2022 Adv. Energy Mater. 12 2201801 |
| [29] | Holoubek J, Yin Y, Li M, Yu M, Meng Y S, Liu P, and Chen Z 2019 Angew. Chem. Int. Ed. 58 18892 |
| [30] | Li Z, Yao Y, Sun S, Jin C, Yao N, Yan C, and Zhang Q 2023 Angew. Chem. Int. Ed. n/a e202303888 |
| [31] | Nan B, Chen L, Rodrigo N D et al. 2022 Angew. Chem. Int. Ed. 61 e202205967 |
| [32] | Yang Y, Chen Y, Tan L, Zhang J, Li N, Ji X, and Zhu Y 2022 Angew. Chem. Int. Ed. 61 e202209619 |
| [33] | Yao Y X, Yao N, Zhou X R, Li Z H, Yue X Y, Yan C, and Zhang Q 2022 Adv. Mater. 34 2206448 |
| [34] | Yin Y J, Yang Y C, Cheng D Y et al. 2022 Nat. Energy 7 548 |
| [35] | Yaakov D, Gofer Y, Aurbach D, and Halalay I C 2010 J. Electrochem. Soc. 157 A1383 |
| [36] | Chekushkin P M, Merenkov I S, Smirnov V S, Kislenko S A, and Nikitina V A 2021 Electrochim. Acta 372 137843 |
|
|
Viewed |
|
|
|
Full text
|
|
|
|
|
Abstract
|
|
|
|
|