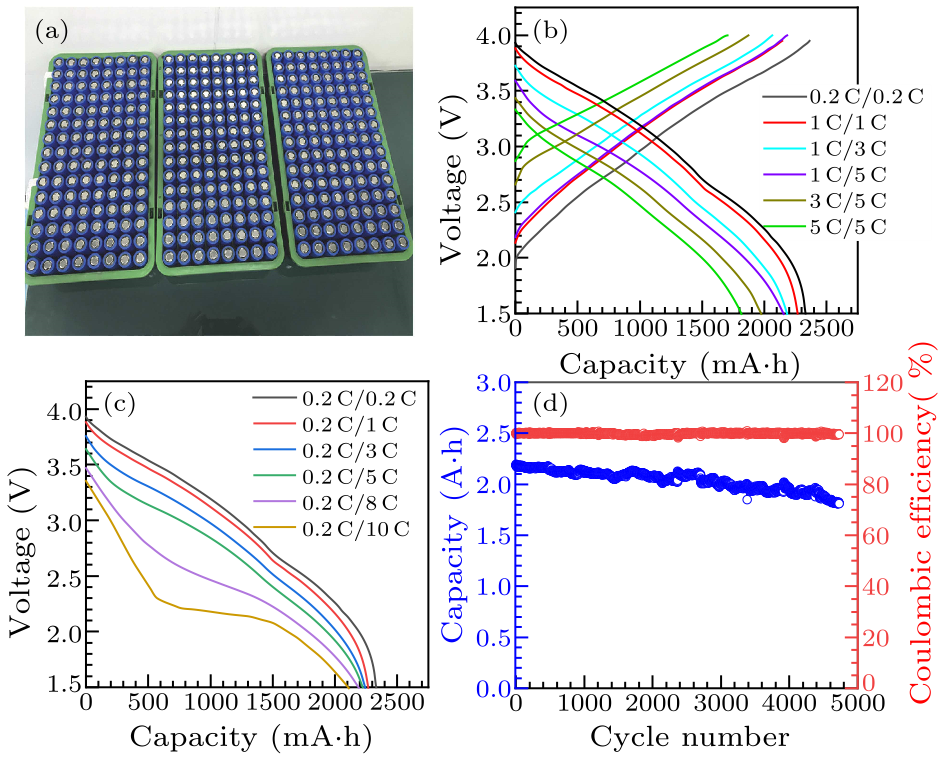
Fig. 1. (a) The high power 26650-type cylindrical NIBs. [(b), (c)] Charge-discharge curves at different rates of high power 26650-type cylindrical NIBs. (d) Cycle performance of high power 26650-type cylindrical NIBs at 2 C/2 C rate.
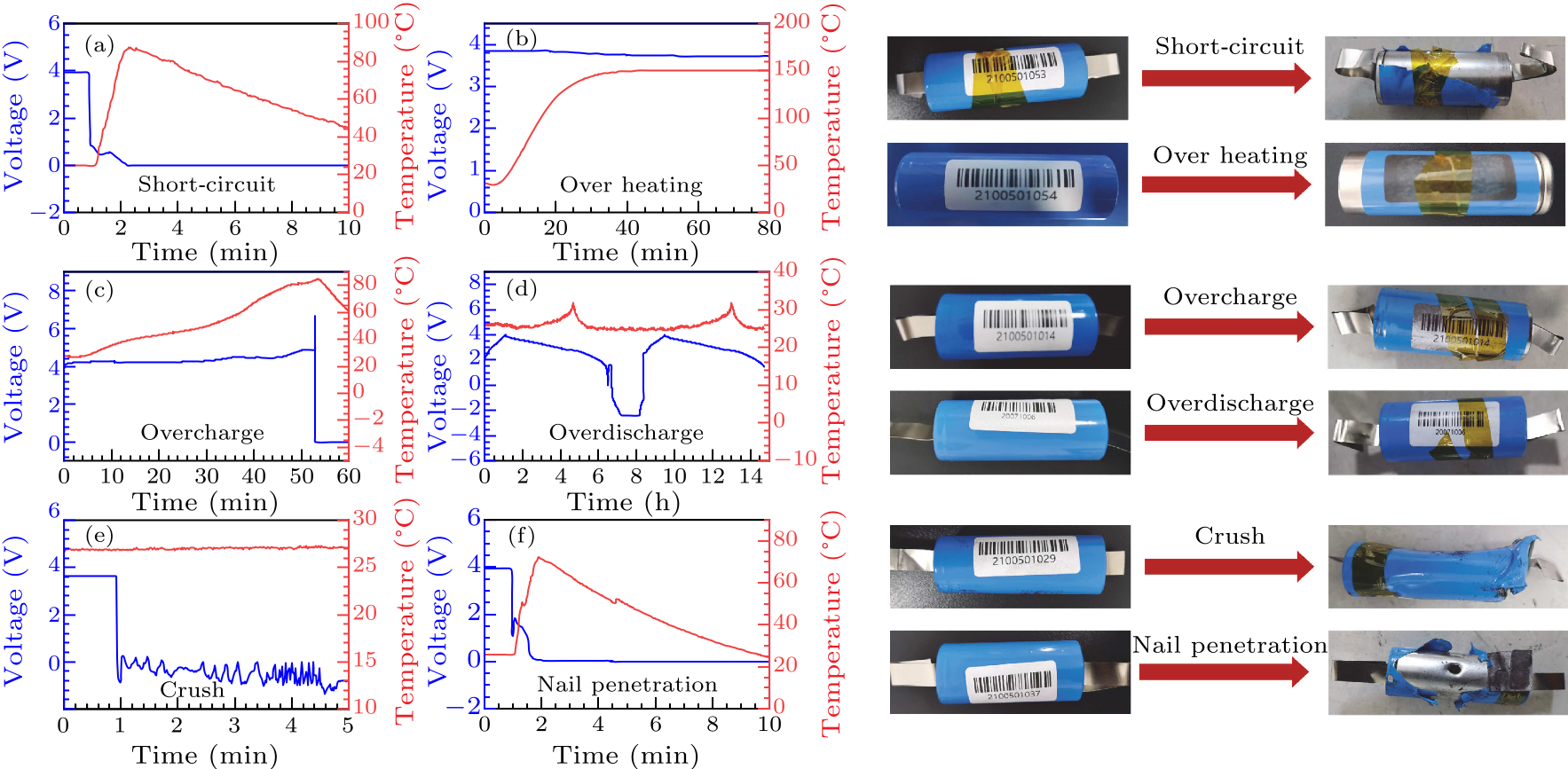
Fig. 2. The safety test results of high power 26650-type cylindrical NIBs at fully charged state. The voltage and temperature evolutions in the process of (a) external short-circuit test, (b) over heating test, (c) overcharge test, (d) overdischarge test, (e) crush tests, and (f) nail penetration test.
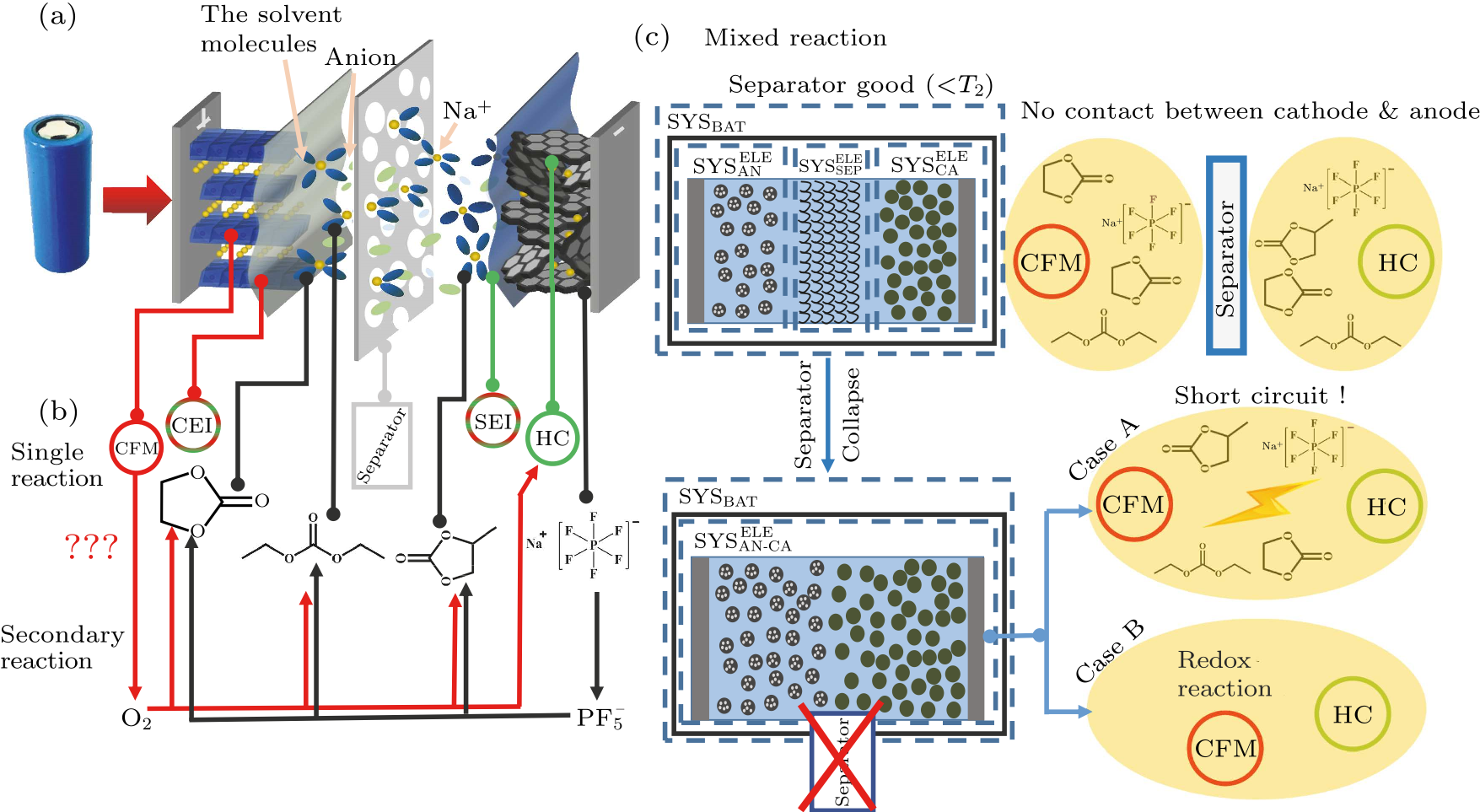
Fig. 3. Untangling the “reaction pathways” based on current understanding. (a) The working principle model of NIBs, with CFM |1 M NaPF$_{6}$/EC + PC + DEC|HC. (b) Single reactions for this battery chemistry, and secondary reactions caused by intermediate products generated from the single reactions. (c) Typical mixed reactions that occur under real world conditions. Further development may lead to the sharp temperature rise. The cases are categorized by the integrity of the separator.[18,35,38]
| Batteries | $T_{1}$ (℃) | $T_{2}$ (℃) | $T_{3}$ (℃) |
|---|---|---|---|
| 100%SOH battery | 110.14 | 198.46 | 349.87 |
| 80%SOH battery | 101.82 | 186.53 | 276.15 |
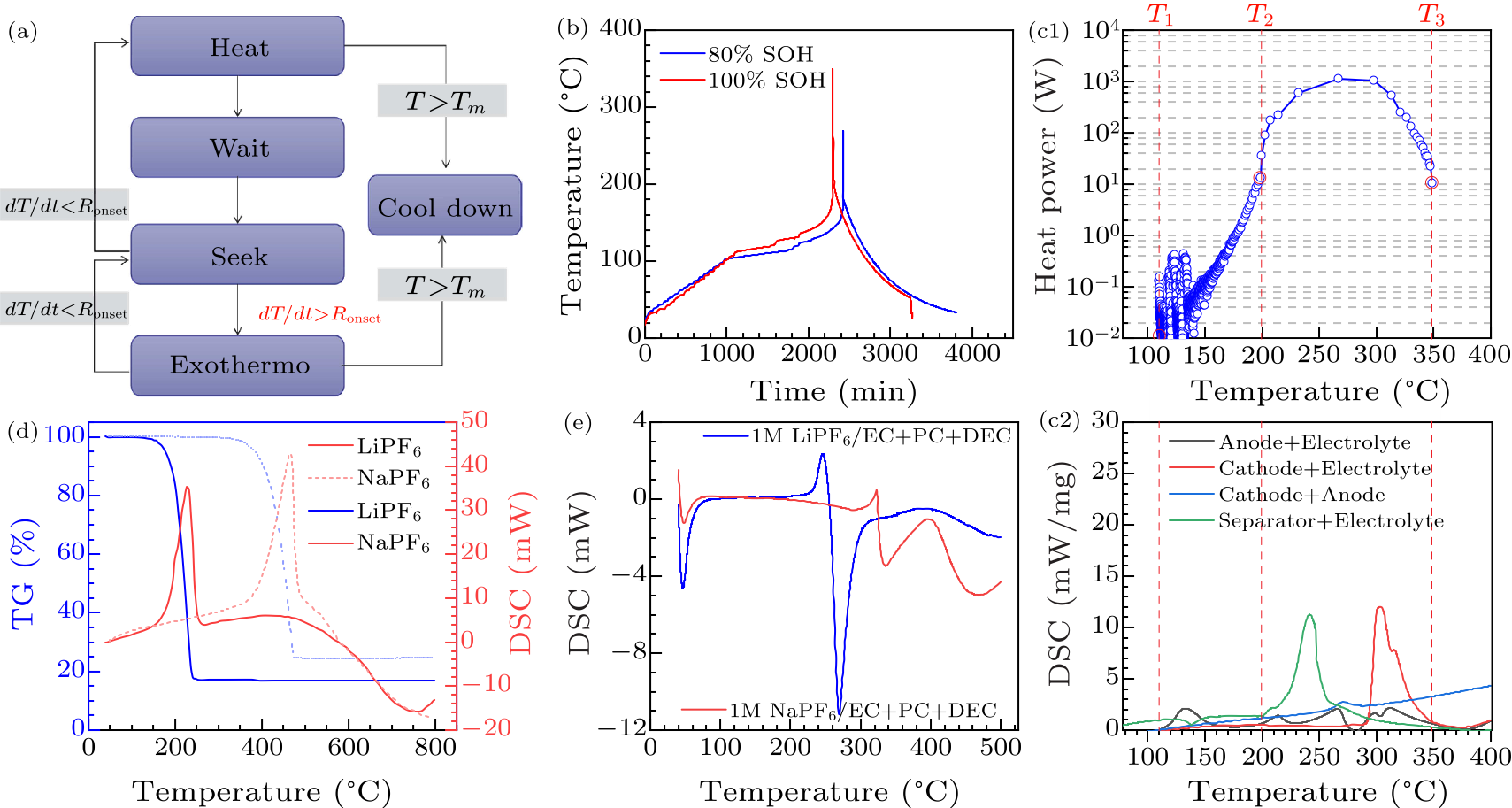
Fig. 4. (a) Heat-wait-seek (HWS) operating mode of ARC, where the Ronset is the set as 0.02 ℃/min, and the maximum test temperature is 350 ℃.[14,39] (b) The time-dependent temperature curves of ARC test of fresh and aged batteries. (c) Interpretation of the heat generation mechanisms. Comparison of the total heat generation power results from the ARC and DSC tests. (d) Comparison of the thermal stability of NaPF$_{6}$ and LiPF$_{6}$. (e) Comparison of the thermal stability of 1 M NaPF$_{6}$/EC + PC + DEC and 1 M LiPF$_{6}$/EC + PC + DEC.
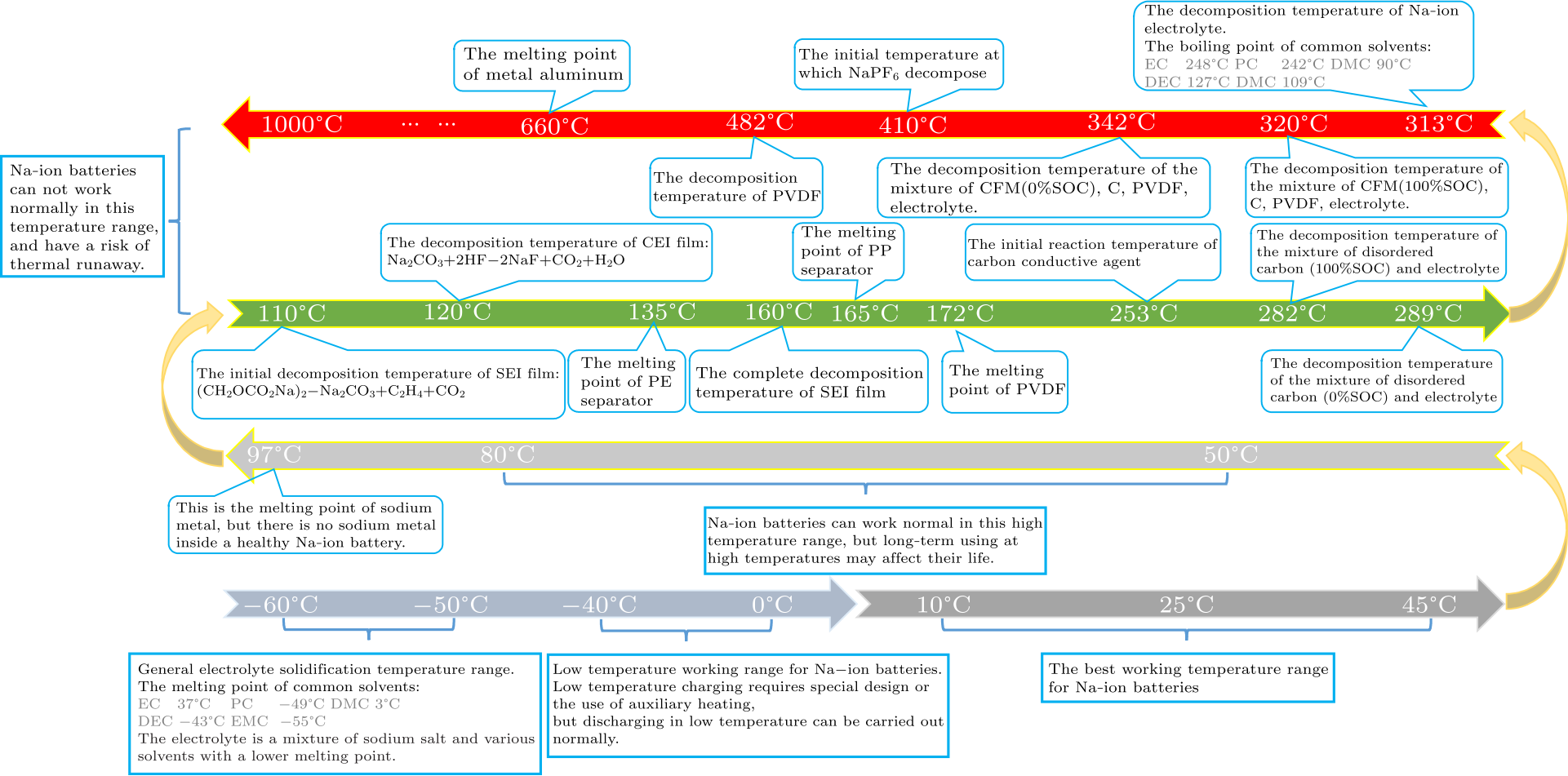
Fig. 5. The whole temperature characteristics of the NIBs from $-60$ ℃ to 1000 ℃.
| [1] | Pan H, Hu Y S, and Chen L 2013 Energy & Environ. Sci. 6 2338 | Room-temperature stationary sodium-ion batteries for large-scale electric energy storage
| [2] | Meng Q, Lu Y, Ding F, Zhang Q, Chen L, and Hu Y S 2019 ACS Energy Lett. 4 2608 | Tuning the Closed Pore Structure of Hard Carbons with the Highest Na Storage Capacity
| [3] | Jiang L, Lu Y, Wang Y, Liu L, Qi X, Zhao C, Chen L, and Hu Y S 2018 Chin. Phys. Lett. 35 048801 | A High-Temperature $\beta$-Phase NaMnO$_{2}$ Stabilized by Cu Doping and Its Na Storage Properties
| [4] | Zhang B, Camélia M, Christel L, Cathie V G, and Tarascon J M 2016 Adv. Energy Mater. 6 1501588 | Correlation Between Microstructure and Na Storage Behavior in Hard Carbon
| [5] | Lei Y, Yan Z, Lai W, Chou S, Wang Y, Liu H, and Dou S 2020 Electrochem. Energy Rev. 3 766 | Tailoring MXene-Based Materials for Sodium-Ion Storage: Synthesis, Mechanisms, and Applications
| [6] | Li Y, Lu Y, Adelhelm P, Titirici M M, and Hu Y S 2019 Chem. Soc. Rev. 48 4655 | Intercalation chemistry of graphite: alkali metal ions and beyond
| [7] | Abraham K M 2020 ACS Energy Lett. 5 3544 | How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts?
| [8] | Chen Z, Xiong R, Lu J, and Li X 2018 Appl. Energy 213 375 | Temperature rise prediction of lithium-ion battery suffering external short circuit for all-climate electric vehicles application
| [9] | DP F, Scheel M, JB R, Tjaden B, Hunt I, and TJ M 2015 Nat. Commun. 6 6924 | In-operando high-speed tomography of lithium-ion batteries during thermal runaway
| [10] | Feng X, Fang M, He X, Ouyang M, Lu L, Wang H, and Zhang M 2014 J. Power Sources 255 294 | Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry
| [11] | MacNeil D D, Larcher D D, and Dahn J R 2019 J. Electrochem. Soc. 146 3596 | Comparison of the Reactivity of Various Carbon Electrode Materials with Electrolyte at Elevated Temperature
| [12] | Lei B, Zhao W, Ziebert C, Uhlmann N, Rohde M, and Seifert H 2017 Batteries 3 14 | Experimental Analysis of Thermal Runaway in 18650 Cylindrical Li-Ion Cells Using an Accelerating Rate Calorimeter
| [13] | Zheng S, Wang L, Feng X, and He X 2018 J. Power Sources 378 527 | Probing the heat sources during thermal runaway process by thermal analysis of different battery chemistries
| [14] | Feng X, Zheng S, He X, Wang L, Wang Y, and Ren D 2018 Front. Energy Res. 6 126 | Time Sequence Map for Interpreting the Thermal Runaway Mechanism of Lithium-Ion Batteries With LiNixCoyMnzO2 Cathode
| [15] | Ren D, Liu X, Feng X, Lu L, Ouyang M, and Li J 2018 Appl. Energy 228 633 | Model-based thermal runaway prediction of lithium-ion batteries from kinetics analysis of cell components
| [16] | Roth E P and Doughty D H 2004 J. Power Sources 128 308 | Thermal abuse performance of high-power 18650 Li-ion cells
| [17] | Li Y, Yang Y, Lu Y, Zhou Q, Qi X, Meng Q, Rong X, Chen L, and Hu Y S 2020 ACS Energy Lett. 5 1156 | Ultralow-Concentration Electrolyte for Na-Ion Batteries
| [18] | Feng X, Zhen S, Ren D, He X, Wang L, Cui H, Liu X, Jin C, Zhang F, Xu C, Hsu H, Gao S, Chen T, Li Y, Wang T, Wang H, Li M, and Ouyang M 2019 Appl. Energy 246 53 | Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database
| [19] | Andersson A M, Edstrom K, Rao N, and Wendsjö Å 1999 J. Power Sources 81–82 286 | Temperature dependence of the passivation layer on graphite
| [20] | Andersson A M, Edstrom K, and Thomas J O 1999 J. Power Sources 81–82 8 | Characterisation of the ambient and elevated temperature performance of a graphite electrode
| [21] | Lee H, W, and Wang Y 2004 J. Electrochem. Soc. 151 A542 | Thermal Stability of the Solid Electrolyte Interface on Carbon Electrodes of Lithium Batteries
| [22] | Sacken U, Nodwell E, Sundher A, and Dahn J 1995 J. Power Sources 54 240 | Comparative thermal stability of carbon intercalation anodes and lithium metal anodes for rechargeable lithium batteries
| [23] | Yang H, Bang H, Amine K, and Prakash J 2005 J. Electrochem. Soc. 152 A73 | Investigations of the Exothermic Reactions of Natural Graphite Anode for Li-Ion Batteries during Thermal Runaway
| [24] | Liu L, Qi X, Yin S, Zhang Q, Liu X, Suo L, Li H, Chen L, and Hu Y S 2019 ACS Energy Lett. 4 1650 | In Situ Formation of a Stable Interface in Solid-State Batteries
| [25] | Aurbach D, Zaban A, Ein-Eli Y, Weissman I, Chusid O, Markovsky B, Levi M, Levi E, Schechter A, and Granot E 1997 J. Power Sources 68 91 | Recent studies on the correlation between surface chemistry, morphology, three-dimensional structures and performance of Li and Li-C intercalation anodes in several important electrolyte systems
| [26] | Doughty D and Roth E P 2012 Electrochem. Soc. Interface 21 37 | A General Discussion of Li Ion Battery Safety
| [27] | Richard M and Dahn J R 1999 J. Electrochem. Soc. 146 2068 | Accelerating Rate Calorimetry Study on the Thermal Stability of Lithium Intercalated Graphite in Electrolyte. I. Experimental
| [28] | Maleki H, Deng G, Anani A, and Howard J 1999 J. Electrochem. Soc. 146 3224 | Thermal Stability Studies of Li‐Ion Cells and Components
| [29] | Yamaki J, Baba Y, Katayama N, Takatsuji H, Egashira M, and Okada S 2003 J. Power Sources 119–121 789 | Thermal stability of electrolytes with LixCoO2 cathode or lithiated carbon anode
| [30] | Qiao R, Zhang M, Liu Y, Ren W, Lin Y, and Pan F 2016 Chin. Phys. Lett. 33 078201 | A Novel Real-Time State-of-Health and State-of-Charge Co-Estimation Method for LiFePO 4 Battery
| [31] | Dahn J R, Fuller E W, Obrovac M, and Vonsacken U 1994 Solid State Ionics 69 265 | Thermal stability of LixCoO2, LixNiO2 and λ-MnO2 and consequences for the safety of Li-ion cells
| [32] | Arai H, Tsuda M, Saito K, Hayashi M, and Sakurai Y 2002 J. Electrochem. Soc. 149 A401 | Thermal Reactions Between Delithiated Lithium Nickelate and Electrolyte Solutions
| [33] | Baba Y, Okada S, and Yamaki J 2002 Solid State Ionics 148 311 | Thermal stability of LixCoO2 cathode for lithium ion battery
| [34] | Ouyang C, Shi S, Wang Z, Li H, Huang X, and Chen L 2005 Chin. Phys. Lett. 22 489 | Temperature-Dependent Dynamic Properties of Li x Mn 2 O 4 in Monte Carlo Simulations
| [35] | Li Y, Lu Y, Meng Q, Jensen A, Zhang Q, Zhang Q, Tong Y, Qi Y, Gu L, Titirici M M, and Hu Y S 2019 Adv. Energy Mater. 9 1902852 | Regulating Pore Structure of Hierarchical Porous Waste Cork‐Derived Hard Carbon Anode for Enhanced Na Storage Performance
| [36] | Liu K, Liu Y, Lin D, Pei A, and Cui Y 2018 Sci. Adv. 4 eaas9820 | Materials for lithium-ion battery safety
| [37] | Larsson F and Mellander B E 2014 J. Electrochem. Soc. 161 A1611 | Abuse by External Heating, Overcharge and Short Circuiting of Commercial Lithium-Ion Battery Cells
| [38] | Xie Y, Xu G, Che H, Wang H, Yang K, Yang X G F, Yang R, Che Z, and Khalil A 2018 Chem. Mater. 30 4909 | Probing Thermal and Chemical Stability of Na x Ni 1/3 Fe 1/3 Mn 1/3 O 2 Cathode Material toward Safe Sodium-Ion Batteries
| [39] | Chen R, Nolan A M, Lu J, Chen L, Huang X, and Li H 2020 Joule 4 812 | The Thermal Stability of Lithium Solid Electrolytes with Metallic Lithium
| [40] | Liu X, Ren D, Hsu H, Feng X, GL X, Zhuang M G H, Lu L, Han X, Chu Z, Li J, He X, Amine K, and Ouyang M 2018 Joule 2 2047 | Thermal Runaway of Lithium-Ion Batteries without Internal Short Circuit
| [41] | Zhang L, Ma Y, Cheng X, Cui Y, Guan T, Gao Y, Du C, Yin G, Lin F, and Nordlund D 2016 J. Power Sources 329 255 | Degradation mechanism of over-charged LiCoO2/mesocarbon microbeads battery during shallow depth of discharge cycling
| [42] | Eshetu G G, Grugeon S, Kim H, Jeong S, Wu L, Gachot G, Laruelle S, Armand M, and Passerini S 2016 ChemSusChem 9 462 | Comprehensive Insights into the Reactivity of Electrolytes Based on Sodium Ions
| [43] | Ponrouch A, Marchante E, Courty M, Tarascon J M, and Palacín M R 2012 Energy & Environ. Sci. 5 8572 | In search of an optimized electrolyte for Na-ion batteries
| [44] | Li Y, Lu Y, Chen L, and Hu Y S 2020 Chin. Phys. B 29 048201 | Failure analysis with a focus on thermal aspect towards developing safer Na-ion batteries
| [45] | Zhu X B and Wang L Z 2020 EcoMat 2 e12043 | Advances in materials for all‐climate sodium‐ion batteries
| [46] | Lee Y, Lim H, Kim S O, Kim H S, Kim K J, Lee K Y, and Choi W 2018 J. Mater. Chem. A 6 20383 | Thermal stability of Sn anode material with non-aqueous electrolytes in sodium-ion batteries
| [47] | Feng J, An Y, Ci L, and Xiong S 2015 J. Mater. Chem. A 3 14539 | Nonflammable electrolyte for safer non-aqueous sodium batteries
| [48] | Hu Y S, Komaba S, Forsyth M, Johnson C, and Rojo T 2019 Small Methods 3 1900184 | A New Emerging Technology: Na‐Ion Batteries