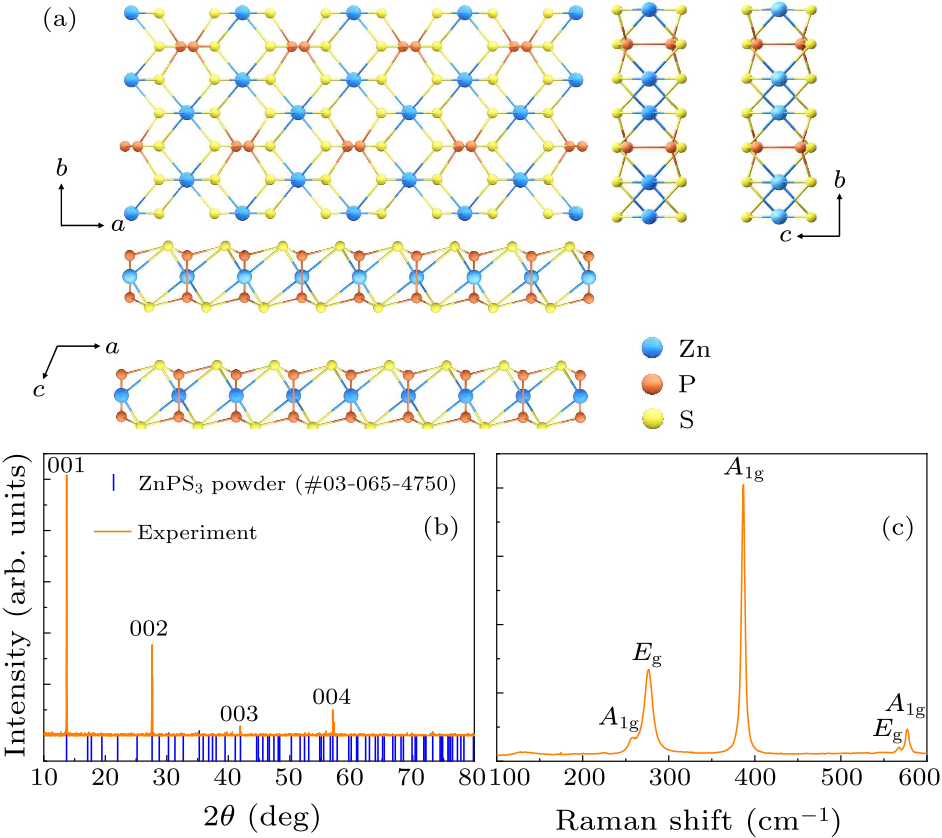
Fig. 1. (a) Schematic crystal structure of ZnPS$_{3}$. Blue, orange and yellow spheres represent Zn, P and S atoms, respectively. (b) Single-crystal XRD pattern of ZnPS$_{3}$. (c) Raman spectrum of ZnPS$_{3}$.
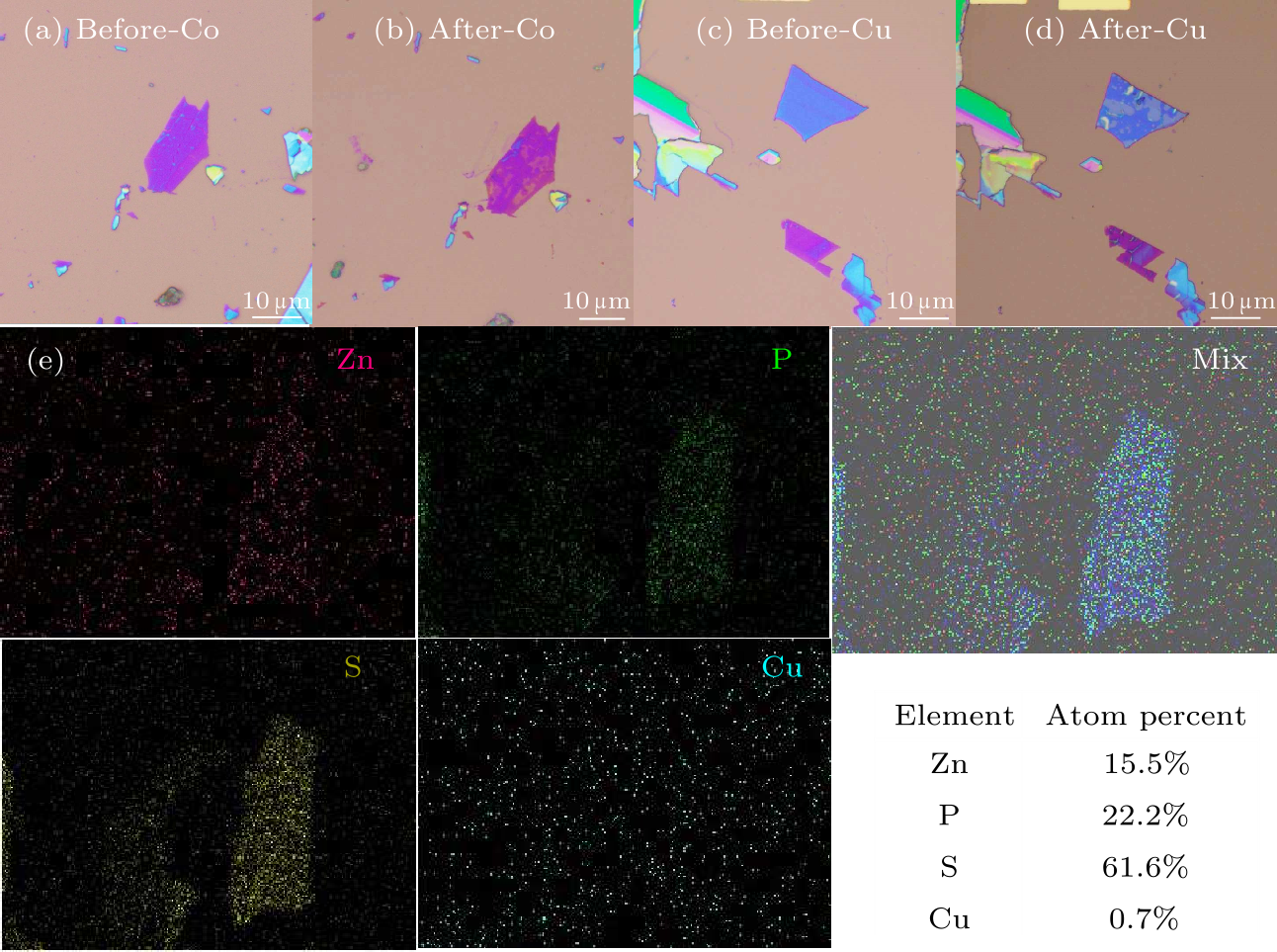
Fig. 2. Optical images of ZnPS$_{3}$ (a) before and (b) after intercalation with Co. Optical images of ZnPS$_{3}$ (c) before and (d) after intercalation with Cu. (e) EDS mapping data of Zn, P, S, Cu and the total for a selected area of the substrate, respectively, and an atomic ratio table taken on the Cu-intercalated flake.
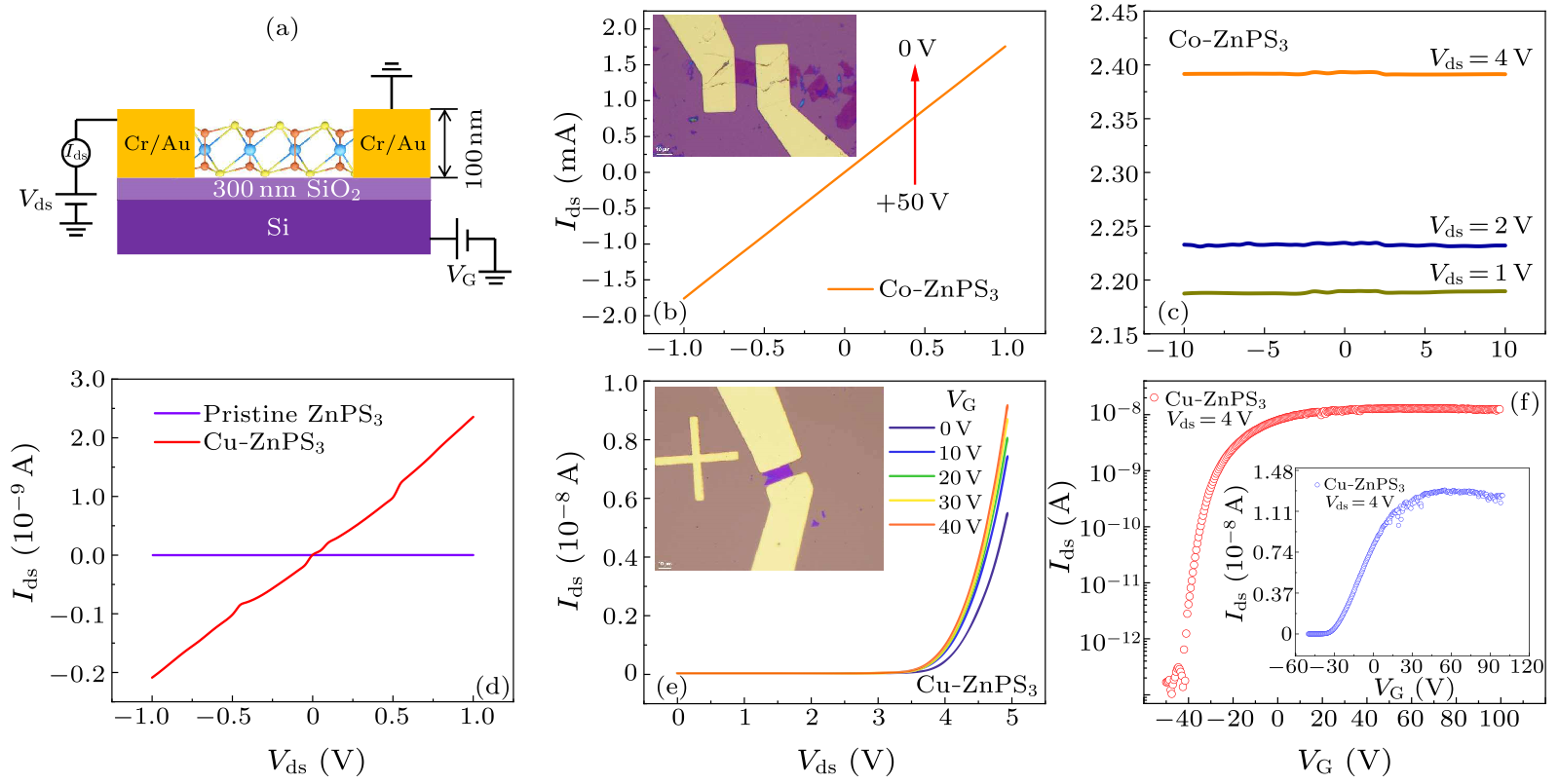
Fig. 3. (a) Cross-section view of the intercalated ZnPS$_{3}$ FET devices. (b) $I_{\rm ds}$–$V_{\rm ds}$ output curves of Co-ZnPS$_{3}$ FET with $V_{\rm G}$ varied from 0 to 50 V. Inset: optical image of Co-ZnPS$_{3}$ device (scale bar is 10 µm). (c) $I_{\rm ds}$–$V_{\rm G}$ transfer curve of Co-ZnPS$_{3}$ FET device with $V_{\rm ds} = 1$ V, 2 V and 4 V, respectively. (d) $I_{\rm ds}$–$V_{\rm ds}$ curves for pristine ZnPS$_{3}$ crystal and Cu-ZnPS$_{3}$, respectively. (e) $I_{\rm ds}$–$V_{\rm ds}$ curves of Cu-ZnPS$_{3}$ FET with $V_{\rm G}$ varied from 0 to 40 V. Inset: optical image of Cu-ZnPS$_{3}$ device (scale bar is 10 µm). (f) $I_{\rm ds}$–$V_{\rm G}$ curve in semilog scale of Cu-ZnPS$_{3}$ FET device with $V_{\rm ds} = 4$ V. Inset: normal scale.
| Materials | Method | Electrical conductivity | References |
|---|---|---|---|
| Pristine ZnPS$_{3}$ | CVT | Insulator | |
| Co-ZnPS$_{3}$ | Solvent method | $\sim$$8\times 10^{4}$ S/m | This work |
| Graphene | Hydrogen arc discharge | $\sim$$2\times 10^{5}$ S/m | |
| 1T-MoS$_{2}$ | Chemical exfoliation | $\sim$$10^{3}$–10$^{4}$ S/m | |
| Antimonene | vdW epitaxial growth | $\sim$$1.6\times 10^{4}$ S/m | |
| 1T-VS$_{2}$ | CVD | $\sim$$3\times 10^{5}$ S/m | |
| Co-SnS$_{2}$ | Solvent method | $\sim$$1.67\times 10^{6}$ S/m | |
| Materials | Method | Type | Carrier mobility (cm$^{2}\cdot$V$^{-1}\cdot$s$^{-1}$) | References | |
|---|---|---|---|---|---|
| Pristine ZnPS$_{3}$ | CVT | Insulator | |
||
| Cu-ZnPS$_{3}$ | Solvent method | n-type | 0.03 | This work | |
| MoS$_{2}$ | Mechanical exfoliation | n-type | 0.1–10 | |
|
| MoS$_{2}$ | CVD on Corning Gorilla Glass | n-type | 0.14 | |
|
| WSe$_{2}$ | CVD | p-type | 0.2 | |
|
| 2H-MoTe$_{2}$ | Mechanical exfoliation | Ambipolar | $0.02$ | |
|
| NiPS$_{3}$ | Mechanical exfoliation | n-type | 0.5–1 | |
|
| MnPS$_{3}$ | Mechanical exfoliation | p-type | $\mathrm{2.7\times }{10}^{-3}$ | |
|
| Cu-SnS$_{2}$ | Solvent method ionic liquid gated | p-type | $40$ | |
|
| [1] | Bae S H et al. 2019 Nat. Mater. 18 550 | Integration of bulk materials with two-dimensional materials for physical coupling and applications
| [2] | Osada M and Sasaki T 2012 Adv. Mater. 24 210 | Two-Dimensional Dielectric Nanosheets: Novel Nanoelectronics From Nanocrystal Building Blocks
| [3] | Gong Y et al. 2018 Nat. Nanotechnol. 13 294 | Spatially controlled doping of two-dimensional SnS2 through intercalation for electronics
| [4] | Susner M A et al. 2017 Adv. Mater. 29 1602852 | Metal Thio- and Selenophosphates as Multifunctional van der Waals Layered Materials
| [5] | Brec R et al. 1987 J. Power Sources 20 205 | Nature of the oxidizing centres in transition metal phosphorus trisulfides MPS3 (M = Fe, Ni)
| [6] | Lacroix P G et al. 1994 Science 263 658 | Stilbazolium-MPS3 Nanocomposites with Large Second-Order Optical Nonlinearity and Permanent Magnetization
| [7] | Ma X et al. 2020 Chem. Commun. 56 4603 | The intercalation of 1,10-phenanthroline into layered NiPS 3 via iron dopant seeding
| [8] | Ichimura K and Sano M 1991 Synth. Met. 45 203 | Electrical conductivity of layered transition-metal phosphorus trisulfide crystals
| [9] | Takano Y et al. 2004 J. Magn. Magn. Mater. 272 E593 | Magnetic properties and specific heat of MPS3 (M=Mn, Fe, Zn)
| [10] | Sourisseau C, Forgerit J P, and Mathey Y 1983 J. Phys. Chem. Solids 44 119 | Vibrational study of layered ZnPS3 compounds intercalated with [Co(η5 -C5H5)2+] and [Cr(η6 -C6H6)2+] CATIONS
| [11] | Boucher F, Evain M, and Brec R 1994 J. Alloys Compd. 215 63 | Second-order Jahn—Teller effect in CdPS3 and ZnPS3 demonstrated by a non-harmonic behaviour of Cd2+ and Zn2+ d10 ions
| [12] | Koski K J et al. 2012 J. Am. Chem. Soc. 134 13773 | Chemical Intercalation of Zerovalent Metals into 2D Layered Bi 2 Se 3 Nanoribbons
| [13] | Yao J et al. 2014 Nat. Commun. 5 5670 | Optical transmission enhacement through chemically tuned two-dimensional bismuth chalcogenide nanoplates
| [14] | Wu Z S et al. 2009 ACS Nano 3 411 | Synthesis of Graphene Sheets with High Electrical Conductivity and Good Thermal Stability by Hydrogen Arc Discharge Exfoliation
| [15] | Prouzet E, Ouvrard G, and Brec R 1986 Mater. Res. Bull. 21 195 | Structure determination of ZnPS3
| [16] | Mayorga-Martinez C C et al. 2017 ACS Appl. Mater. & Interfaces 9 12563 | Layered Metal Thiophosphite Materials: Magnetic, Electrochemical, and Electronic Properties
| [17] | Acerce M, Voiry D, and Chhowalla M 2015 Nat. Nanotechnol. 10 313 | Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials
| [18] | Ji J et al. 2016 Nat. Commun. 7 13352 | Two-dimensional antimonene single crystals grown by van der Waals epitaxy
| [19] | Ji Q et al. 2017 Nano Lett. 17 4908 | Metallic Vanadium Disulfide Nanosheets as a Platform Material for Multifunctional Electrode Applications
| [20] | Radisavljevic B et al. 2011 Nat. Nanotechnol. 6 147 | Single-layer MoS2 transistors
| [21] | Nasr J R et al. 2020 ACS Nano 14 15440 | Low-Power and Ultra-Thin MoS 2 Photodetectors on Glass
| [22] | Huang J et al. 2015 Nanoscale 7 4193 | Large-area synthesis of monolayer WSe 2 on a SiO 2 /Si substrate and its device applications
| [23] | Lin Y F et al. 2015 Adv. Mater. 27 6612 | Origin of Noise in Layered MoTe 2 Transistors and its Possible Use for Environmental Sensors
| [24] | Lin Y F et al. 2014 Adv. Mater. 26 3263 | Ambipolar MoTe 2 Transistors and Their Applications in Logic Circuits
| [25] | Jenjeti R T et al. 2018 Sci. Rep. 8 8586 | Field Effect Transistor Based on Layered NiPS 3
| [26] | Kumar R et al. 2019 J. Mater. Chem. C 7 324 | Bulk and few-layer MnPS 3 : a new candidate for field effect transistors and UV photodetectors
| [27] | Jena D and Konar A 2007 Phys. Rev. Lett. 98 136805 | Enhancement of Carrier Mobility in Semiconductor Nanostructures by Dielectric Engineering
| [28] | Bertolazzi S et al. 2019 Adv. Mater. 31 1806663 | Nonvolatile Memories Based on Graphene and Related 2D Materials