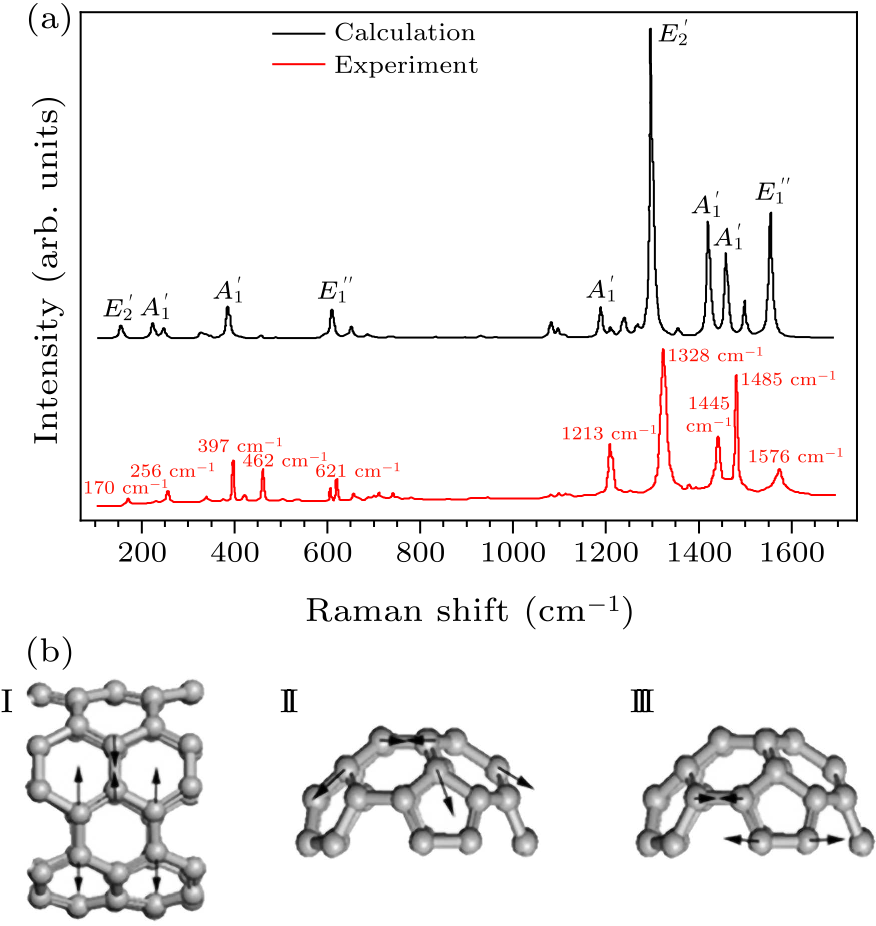

Fig. 1. (a) Experimental and calculated Raman spectra of $D_{5h}$(1)-C$_{90}$ (by Gaussian 09) under ambient conditions. (b) The eigenvectors of Raman vibration modes for four bands at 1213 cm$^{-1}$ (I), 1445 cm$^{-1}$ (II), 1485 cm$^{-1}$ (III), and 1576 cm$^{-1}$ (IV), respectively.

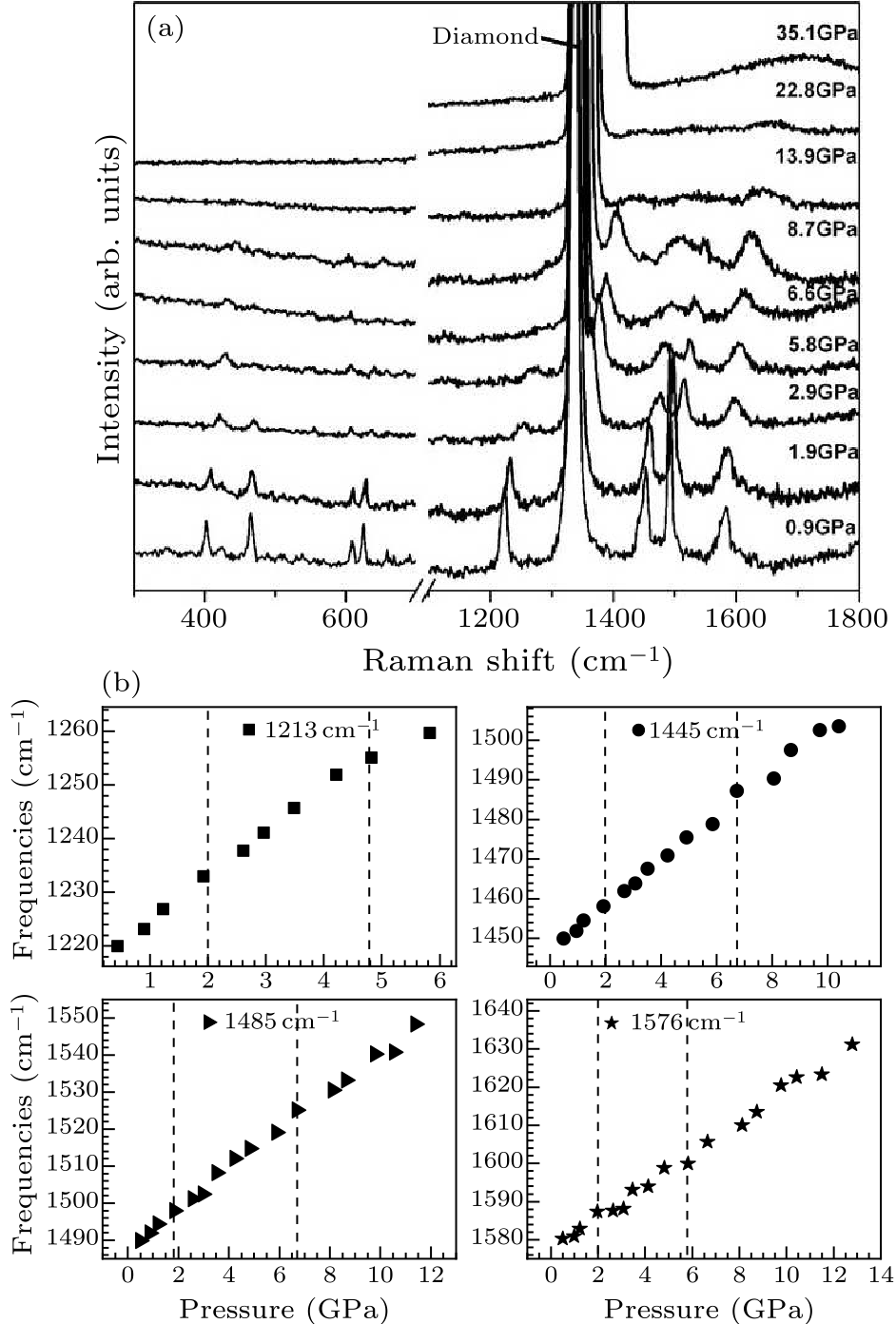
Fig. 2. (a) In situ Raman scattering spectra of $D_{5h}$(1)-C$_{90}$ measured with 514 nm laser excitation at different pressures. (b) Pressure-dependent frequencies of the Raman vibration modes of $D_{5h}$(1)-C$_{90}$ in the pressure regime. The initial positions of the four bands are at 1213 cm$^{-1}$, 1445 cm$^{-1}$, 1485 cm$^{-1}$, and 1576 cm$^{-1}$, respectively.

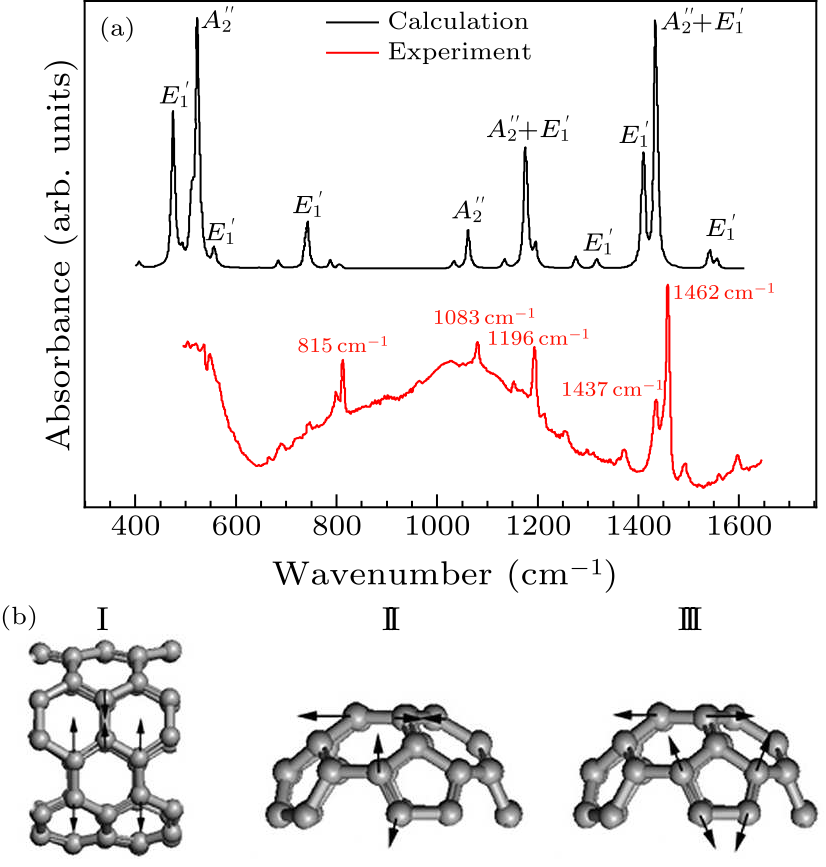
Fig. 3. (a) Experimental and calculated infrared spectra of $D_{5h}$(1)-C$_{90}$ (by Gaussian 09) under ambient conditions. (b) The eigenvectors of IR vibration modes for five bands at 815 cm$^{-1}$ (I), 1083 cm$^{-1}$ (I), 1196 cm$^{-1}$ (I), 1437 cm$^{-1}$ (II), and 1462 cm$^{-1}$ (III), respectively.

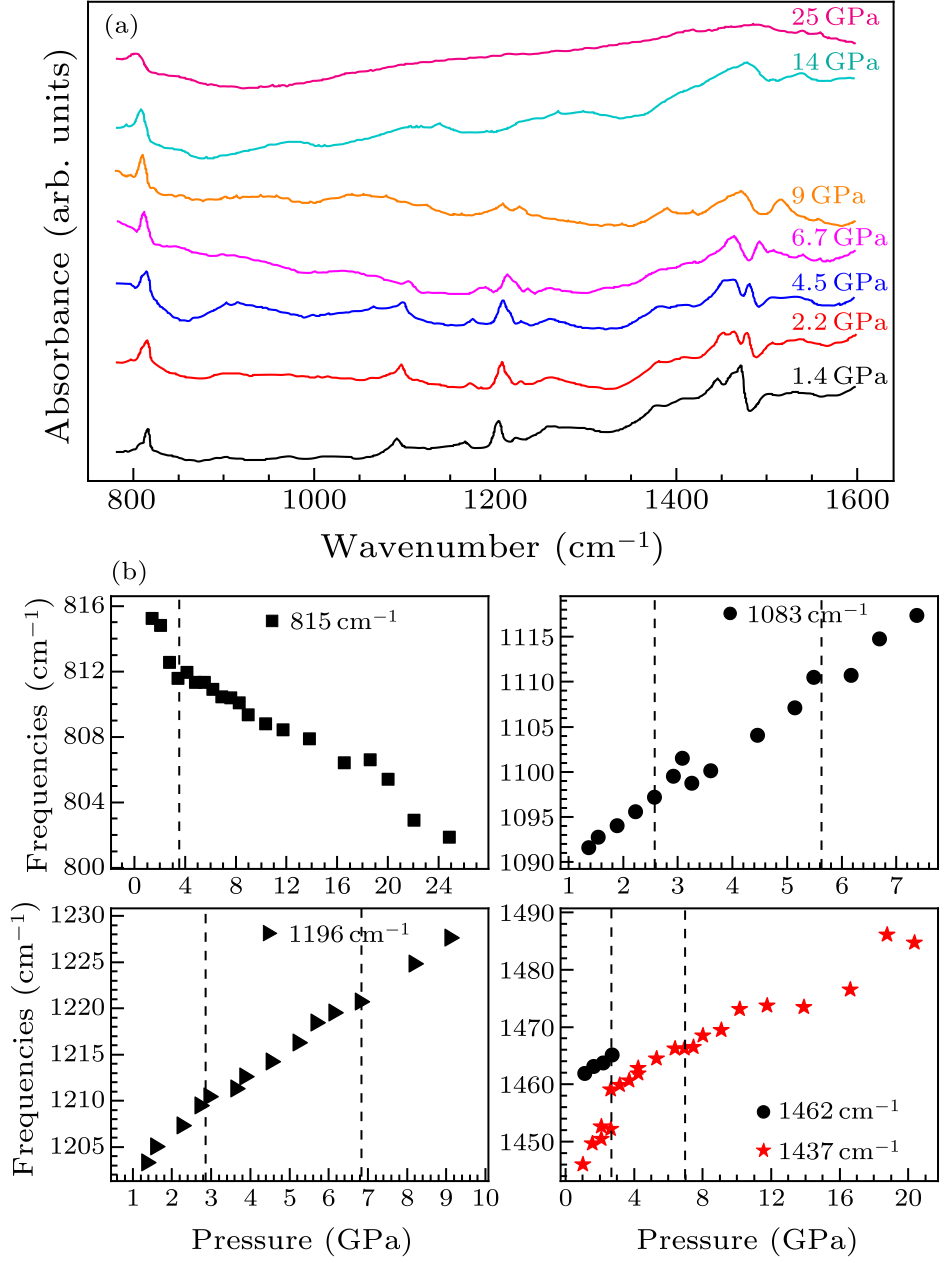
Fig. 4. (a) Mid-IR absorption spectra of $D_{5h}$(1)-C$_{90}$ at different pressures. (b) Pressure dependence of the peak positions for the five selected infrared vibration modes of $D_{5h}$(1)-C$_{90}$. The initial positions of the five bands are at 815 cm$^{-1}$, 1083 cm$^{-1}$, 1196 cm$^{-1}$, 1437 cm$^{-1}$, and 1462 cm$^{-1}$, respectively.

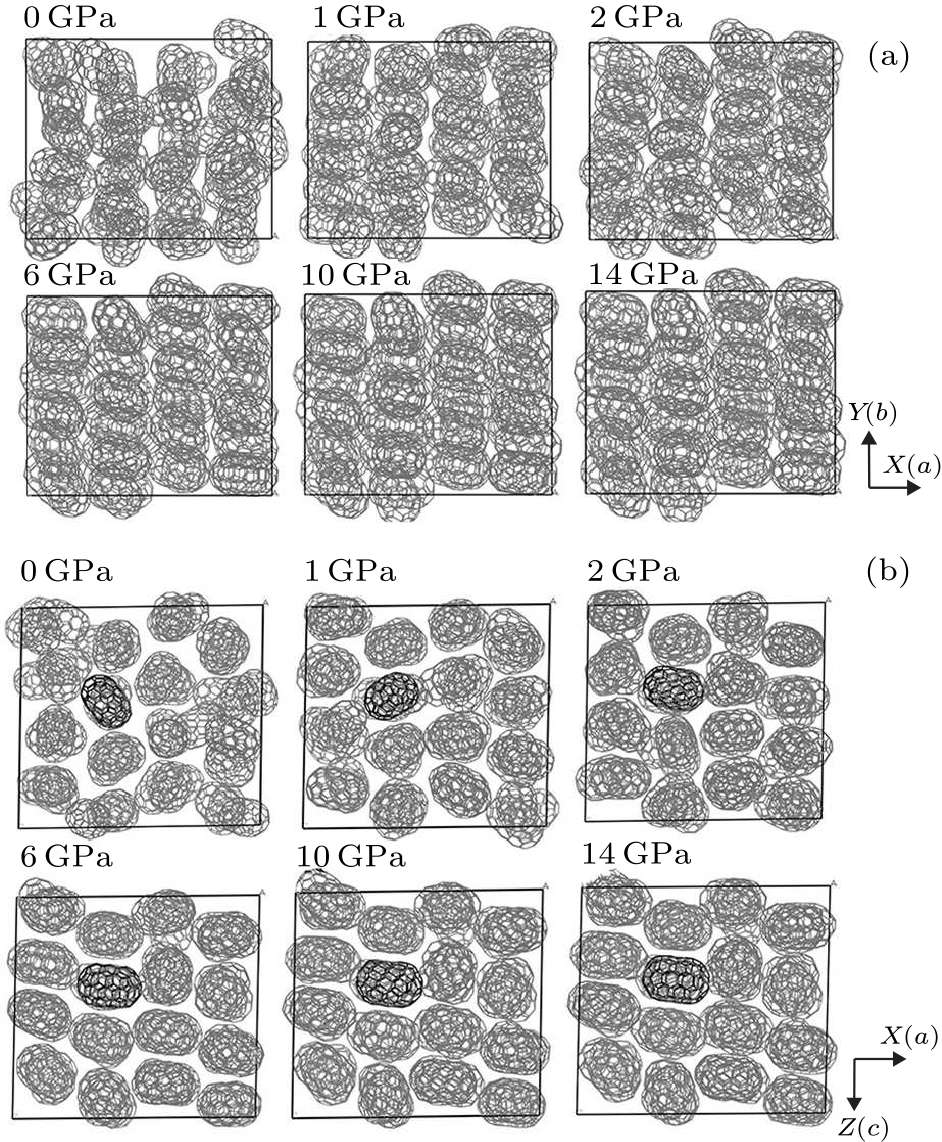
Fig. 5. (a) Orientations of $D_{5h}$(1)-C$_{90}$ at different pressures. (b) The rotations of $D_{5h}$(1)-C$_{90}$ at different pressures. The results are simulated by Materials Studio 5.5.

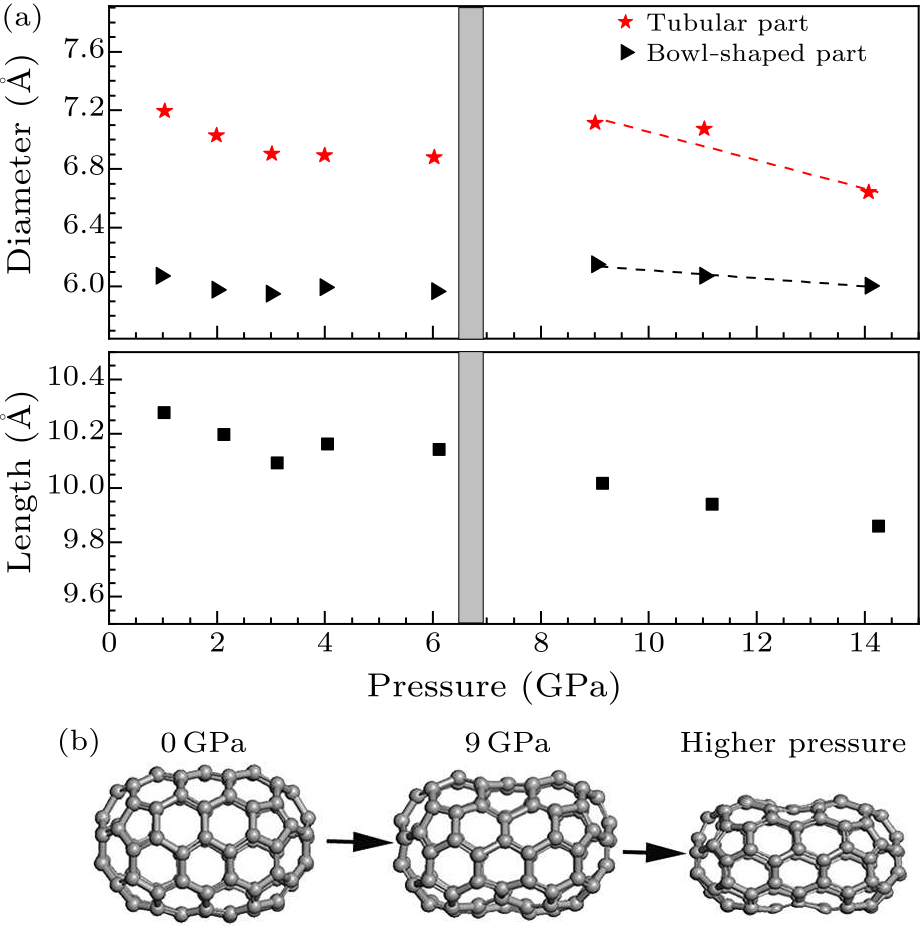
Fig. 6. (a) Pressure dependence of the lengths of $D_{5h}$(1)-C$_{90}$ and the diameters of the tubular and bowl-shaped parts. (b) The deformation process of $D_{5h}$(1)-C$_{90}$ under pressure.
| [1] | Yamanaka S, Kini N S, Kubo A, Jida S, and Kuramoto H 2008 J. Am. Chem. Soc. 130 4303 | Topochemical 3D Polymerization of C60 under High Pressure at Elevated Temperatures
| [2] | Shang Y, Liu Z, Dong J, Yao M, Yang Z, Li Q, Zhai C, Shen F, Hou X, Wang L, Zhang N, Zhang W, Fu R, Ji J, Zhang X, Lin H, Fei Y, Sundqvist B, Wang W, and Liu B 2021 Nature 599 599 | Ultrahard bulk amorphous carbon from collapsed fullerene
| [3] | Zhang S, Li Z, Luo K, He J, Gao Y, Soldatov A V, Benavides V, Shi K, Nie A, Zhang B, Hu W, Ma M, Liu Y, Wen B, Gao G, Liu B, Zhang Y, Shu Y, Yu D, Zhou X F, Zhao Z, Xu B, Su L, Yang G, Chernogorova O P, and Tian Y 2022 Natl. Sci. Rev. 9 nwab140 | Discovery of carbon-based strongest and hardest amorphous material
| [4] | Tang H, Yuan X, Cheng Y, Fei H, Liu F, Liang T, Zeng Z, Ishii T, Wang M S, Katsura T, Sheng H, and Gou H 2021 Nature 599 605 | Synthesis of paracrystalline diamond
| [5] | Wang L, Liu B, Li H, Yang W, Ding Y, Sinogeikin S V, Meng Y, Liu Z, Zeng X C, and Mao W L 2012 Science 337 825 | Long-Range Ordered Carbon Clusters: A Crystalline Material with Amorphous Building Blocks
| [6] | Zhang Y, Yao M, Du M, Yao Z, Wang Y, Dong J, Yang Z, Sundqvist B, Kováts P S, and Liu B 2020 J. Am. Chem. Soc. 142 7584 | Negative Volume Compressibility in Sc3 N@C80 –Cubane Cocrystal with Charge Transfer
| [7] | Elliott J A, Sandler J K W, Windle A H, Young R J, and Shaffer M S P 2004 Phys. Rev. Lett. 92 95501 | Collapse of Single-Wall Carbon Nanotubes is Diameter Dependent
| [8] | Zang J, Treibergs A, Han Y, and Liu F 2004 Phys. Rev. Lett. 92 105501 | Geometric Constant Defining Shape Transitions of Carbon Nanotubes under Pressure
| [9] | Sluiter M H F and Kawazoe Y 2004 Phys. Rev. B 69 224111 | Phase diagram of single-wall carbon nanotube crystals under hydrostatic pressure
| [10] | Araujo P T, Barbosa N N M, Chacham H et al. 2012 Nano Lett. 12 4110 | In Situ Atomic Force Microscopy Tip-Induced Deformations and Raman Spectroscopy Characterization of Single-Wall Carbon Nanotubes
| [11] | Li W, Qu F, Liu L, Zhang Z, Liang J, Lu Y, Zhang J, Wang L, Wang C, and Wang T 2022 Angew. Chem. Int. Ed. 61 e202116854 | A Metallofullertube of Ce2 @C100 with a Carbon Nanotube Segment: Synthesis, Single‐Molecule Conductance and Supramolecular Assembly
| [12] | Ye X, Yu P, Shen W, Hu S, Akasaka T, and Lu X 2021 Sol. RRL 5 2100463 | Er@C82 as a Bifunctional Additive to the Spiro‐OMeTAD Hole Transport Layer for Improving Performance and Stability of Perovskite Solar Cells
| [13] | Wang S, Li X, Zhang X, Huang P, Fang P, Wang J, Yang S, Wu K, and Du P 2021 Chem. Sci. 12 10506 | A supramolecular polymeric heterojunction composed of an all-carbon conjugated polymer and fullerenes
| [14] | Yang H, Jin H, Zhen H, Wang Z, Liu Z, Beavers C M, Mercado B Q, Olmstead M M, and Balch A L 2011 J. Am. Chem. Soc. 133 6299 | Isolation and Crystallographic Identification of Four Isomers of Sm@C90
| [15] | Yang H, Beavers C M, Wang Z, Jiang A, Liu Z, Jin H, Mercado B Q, Olmstead M M, and Balch A L 2010 Angew. Chem. Int. Ed. 49 886 | Isolation of a Small Carbon Nanotube: The Surprising Appearance of D5h(1)-C90
| [16] | Schettino V, Pagliai M, Ciabini L, and Cardini G 2001 J. Phys. Chem. A 105 11192 | The Vibrational Spectrum of Fullerene C60
| [17] | Braga S F and Galvão D S 2007 J. Comput. Chem. 28 1724 | Molecular dynamics simulation of single wall carbon nanotubes polymerization under compression
| [18] | Wang L, Liu B, Liu D, Yao M, Yu S, Hou Y, Zou B, Cui T, Zou G, Sundqvist B, Luo Z, Li H, Li Y, Liu J, Chen S, Wang G, and Liu Y 2007 Appl. Phys. Lett. 91 103112 | Synthesis and high pressure induced amorphization of C60 nanosheets
| [19] | Yamawaki H, Yoshida M, Kakudate Y, Usuba S, Yokoi H, Fujiwara S, Aoki K, Ruoff R, Malhotra R, and Lorents D 1993 J. Phys. Chem. 97 11161 | Infrared study of vibrational property and polymerization of fullerene C60 and C70 under pressure
| [20] | Huang Y, Gilson D F R, and Butler I S 1991 J. Phys. Chem. 95 5723 | High-pressure infrared spectroscopic study of buckminsterfullerene, C60
| [21] | Wagner J, Ramsteiner M, Wild C, and Koidl P 1989 Phys. Rev. B 40 1817 | Resonant Raman scattering of amorphous carbon and polycrystalline diamond films
| [22] | Weiler M, Sattel S, Giessen T, Jung K, Ehrhardt H, Veerasamy V S, and Robertson J 1996 Phys. Rev. B 53 1594 | Preparation and properties of highly tetrahedral hydrogenated amorphous carbon
| [23] | Liu D, Yao M, Wang L, Li Q, Cui W, Liu B, Liu R, Zou B, Cui T, Liu B, Liu J, Sundqvist B, and Wågberg T 2011 J. Phys. Chem. C 115 8918 | Pressure-Induced Phase Transitions of C70 Nanotubes
| [24] | Maksimov A A, Meletov K P, Osip'yan Y A, Tartakovskii I I, Artemov Y V, and Nudel'Man M A 1993 Sov. J. Exp. Theor. Phys. Lett. 57 816 |
| [25] | Rao A M, Menon M, Wang K A, Eklund P C, Subbaswamy K R, Cornett D S, Duncan M A, and Amster I J 1994 Chem. Phys. Lett. 224 106 | Photoinduced polymerization of solid C70 films
| [26] | Thirunavukkuarasu K, Long V C, Musfeldt J L, Borondics F, Klupp G, Kamarás K, and Kuntscher C A 2011 J. Phys. Chem. C 115 3646 | Rotational Dynamics in C70 : Temperature- and Pressure-Dependent Infrared Studies
| [27] | Wasa S, Suito K, Kobayashi M, and Onodera A 2000 Solid State Commun. 114 209 | Pressure-induced irreversible amorphization of C70 fullerene
| [28] | Aguiar A L, Barros E B, Capaz R B, Souza F A G, Freire P T C, Filho J M, Machon D, Caillier C, Kim Y A, Muramatsu H, Endo M, and San-Miguel A 2011 J. Phys. Chem. C 115 5378 | Pressure-Induced Collapse in Double-Walled Carbon Nanotubes: Chemical and Mechanical Screening Effects
| [29] | Yao M, Wang Z, Liu B, Zou Y, Yu S, Lin W, Hou Y, Pan S, Jin M, Zou B, Cui T, Zou G, and Sundqvist B 2008 Phys. Rev. B 78 205411 | Raman signature to identify the structural transition of single-wall carbon nanotubes under high pressure