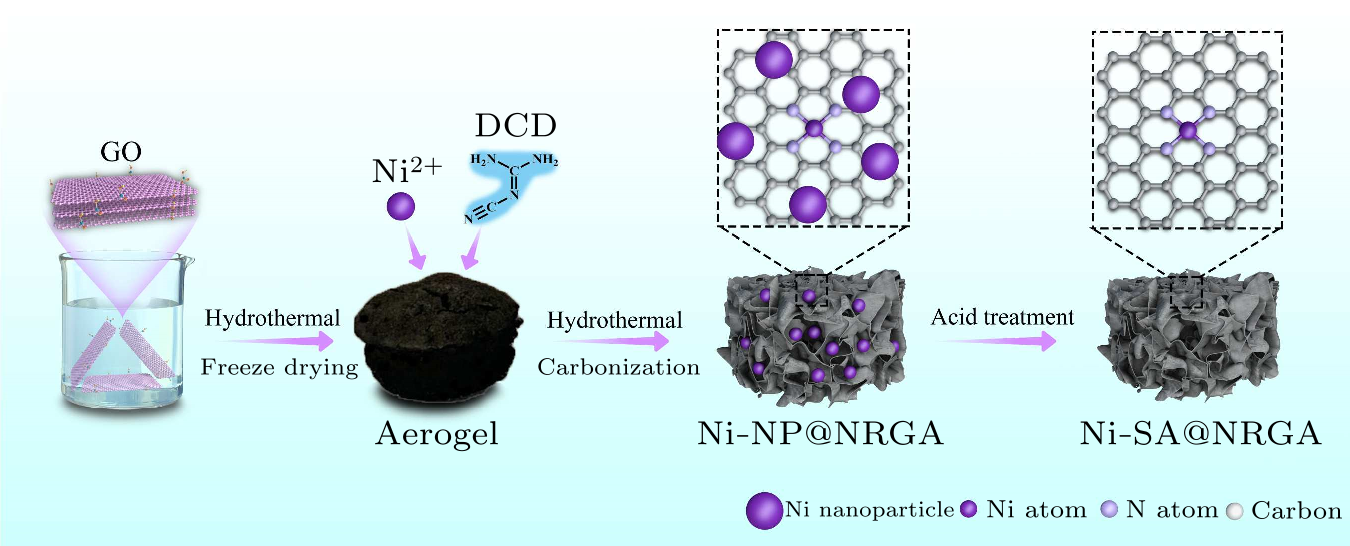

Fig. 1. Schematic illustration of the fabrication process of the Ni-SA@NRGA.

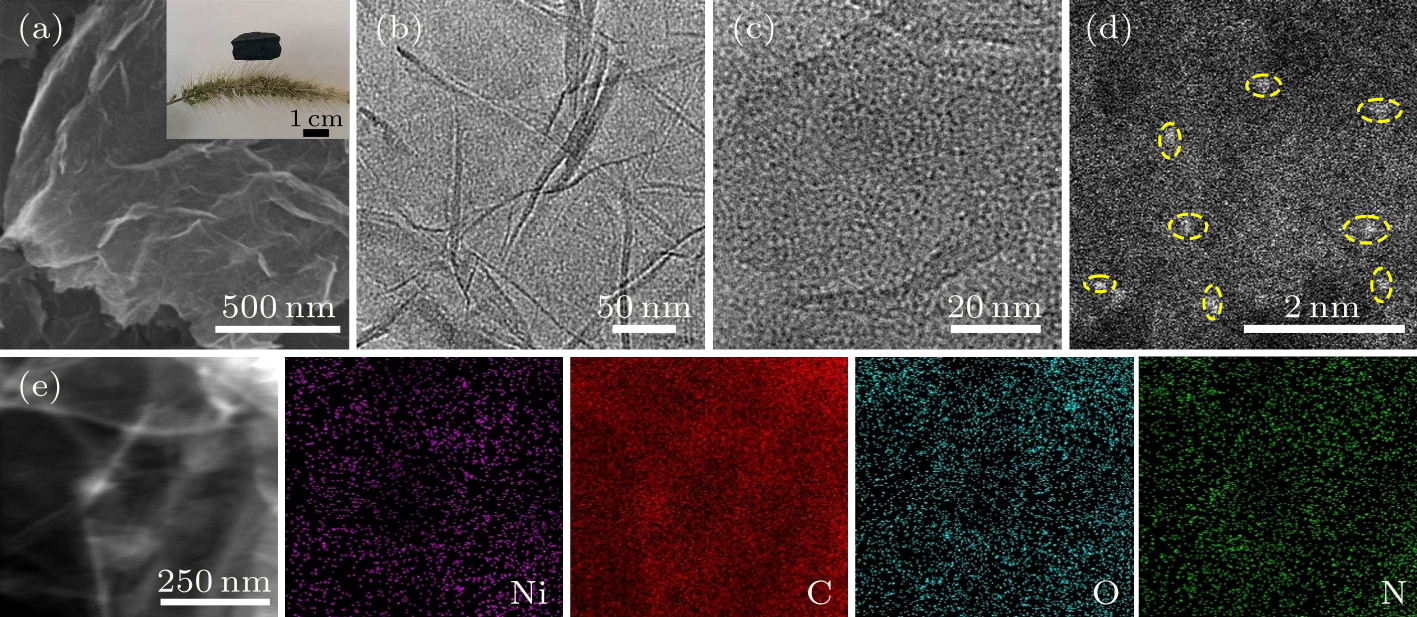
Fig. 2. (a) SEM image of the Ni-SA@NRGA, with the inset showing the digital photograph of Ni-SA@NRGA on the Setaria Viridis. (b) TEM image of the Ni-SA@NRGA. (c) HRTEM image of the Ni-SA@NRGA. (d) AC-HAADF-STEM image of the Ni-SA@NRGA. (e) TEM image and the corresponding EDX Ni, C, O, and N mappings of Ni-SA@NRGA.

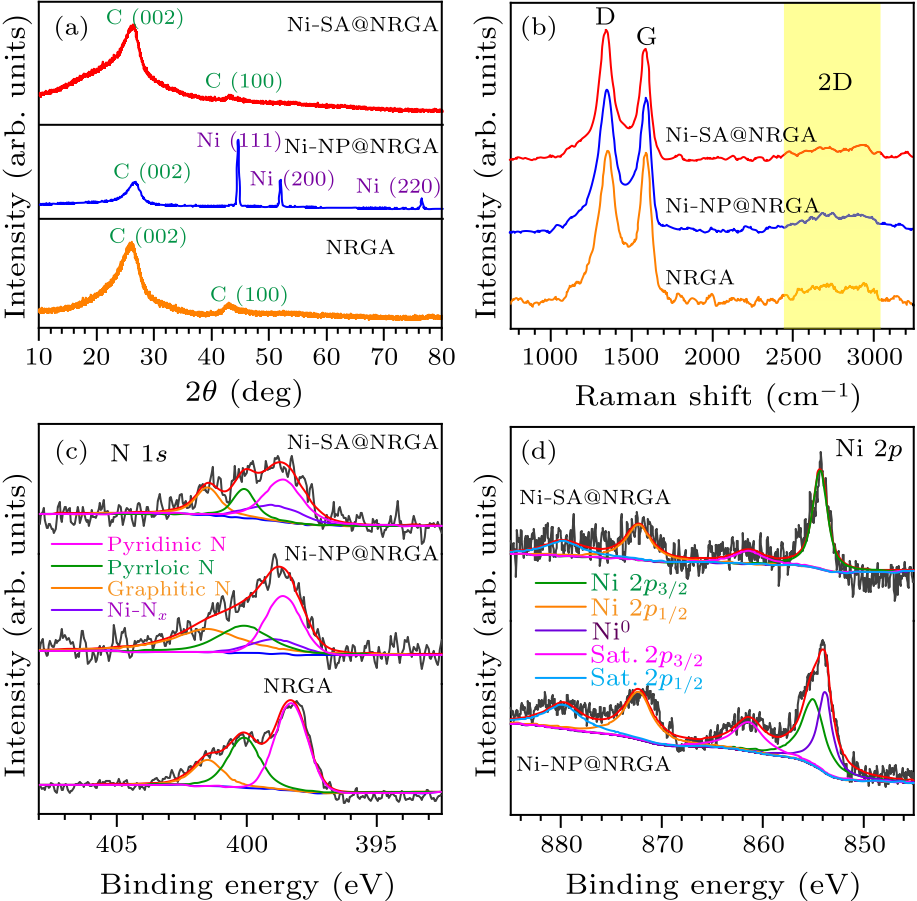
Fig. 3. (a) XRD patterns and (b) Raman spectra of Ni-SA@NRGA, Ni-NP@NRGA, and NRGA. (c) XPS spectra of N $1s$ of NRGA, Ni-SA@NRGA, and Ni-NP@NRGA. (d) XPS spectra of Ni $2p$ of Ni-SA@NRGA and Ni-NP@NRGA.

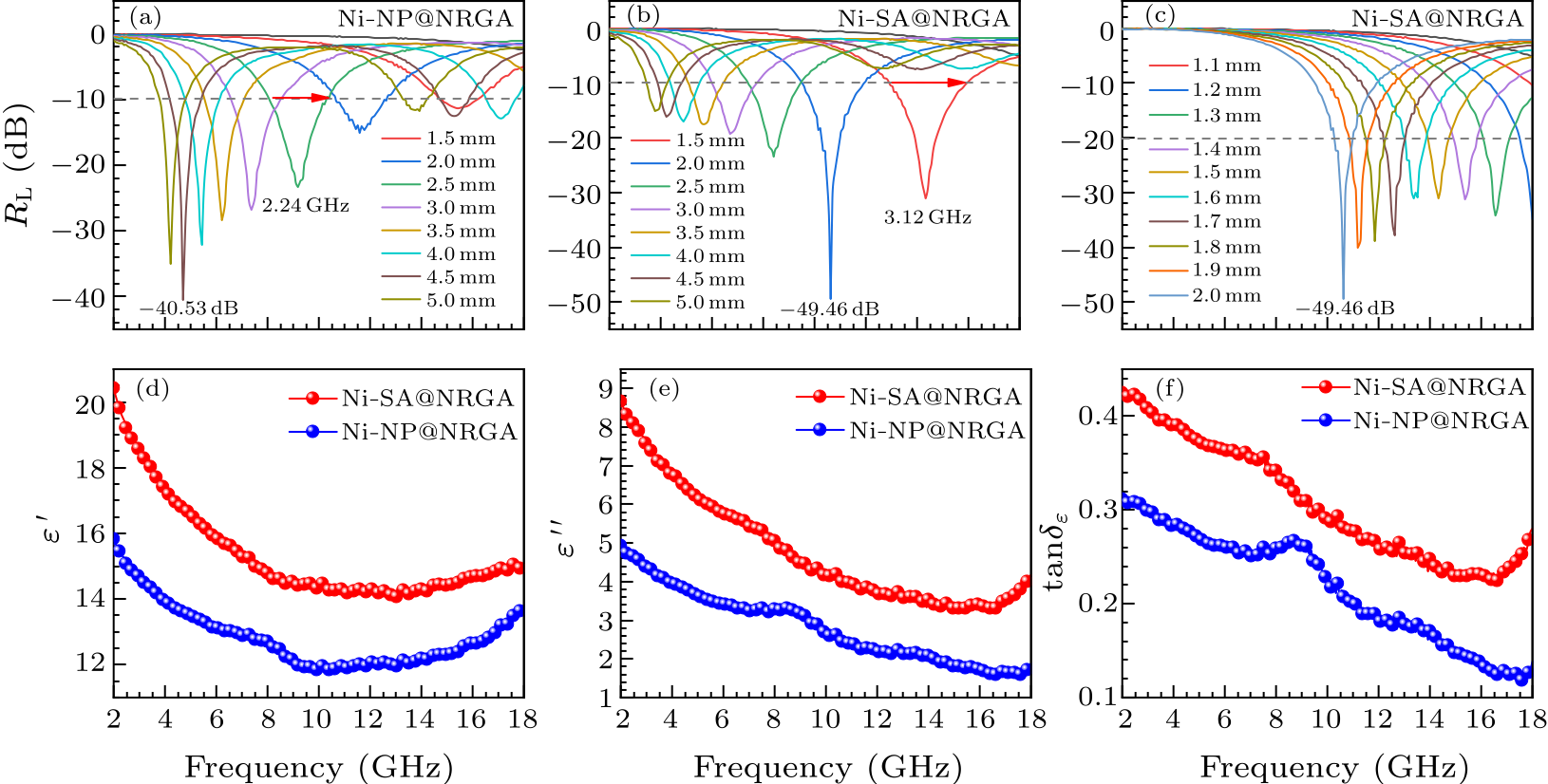
Fig. 4. (a) $R_{\rm L}$–$f$ curves of Ni-NP@NRGA with thickness of 1.5–5.0 mm. (b) $R_{\rm L}$–$f$ curves of Ni-SA@NRGA with thickness of 1.5–5.0 mm. (c) $R_{\rm L}$–$f$ curves of Ni-SA@NRGA with thickness of 1.1–2.0 mm. (d)–(f) Behaviors of $\varepsilon'$, $\varepsilon ''$ and tan$\delta_{\varepsilon}$ of the complex permittivity and loss tangents of the Ni-SA@NRGA and Ni-NP@NRGA samples.

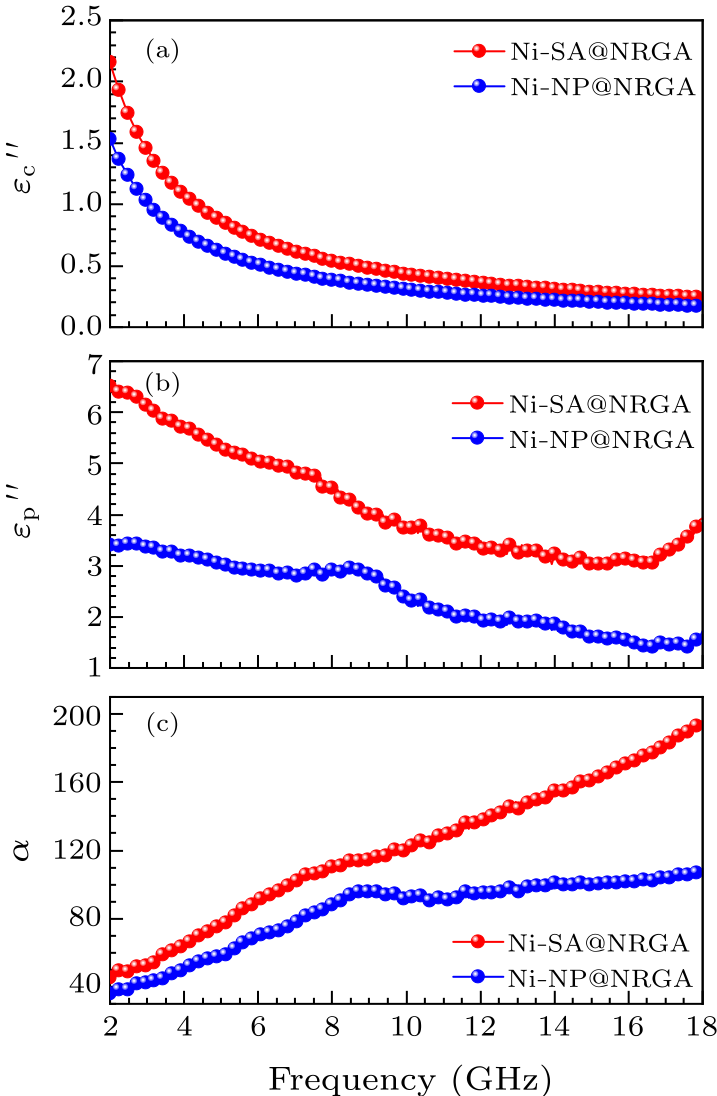
Fig. 5. (a) Conductive loss and (b) polarization loss of Ni-SA@NRGA and Ni-NP@NRGA. (c) The attenuation coefficients of Ni-SA@NRGA and Ni-NP@NRGA.
| [1] | Zhao H Q, Cheng Y, Zhang Z, Zhang B S, Pei C C, Fan F Y, and Ji G B 2021 Carbon 173 501 | Biomass-derived graphene-like porous carbon nanosheets towards ultralight microwave absorption and excellent thermal infrared properties
| [2] | Zhu X, Yan F, Li C Y, Qi L H, Yuan H R, Liu Y F, Zhu C L, and Chen Y J 2021 Chin. Phys. Lett. 38 015201 | Nitrogen and Boron Co-Doped Carbon Nanotubes Embedded with Nickel Nanoparticles as Highly Efficient Electromagnetic Wave Absorbing Materials
| [3] | Wang Z C, Wei R B, Gu J W, Liu H, Liu C T, Luo C J, Kong J, Shao Q, Wang N, Guo Z H, and Liu X B 2018 Carbon 139 1126 | Ultralight, highly compressible and fire-retardant graphene aerogel with self-adjustable electromagnetic wave absorption
| [4] | Zhang X C, Xu J, Yuan H R, Zhang S, Ouyang Q Y, Zhu C L, Zhang X T, and Chen Y J 2019 ACS Appl. Mater. & Interfaces 11 39100 | Large-Scale Synthesis of Three-Dimensional Reduced Graphene Oxide/Nitrogen-Doped Carbon Nanotube Heteronanostructures as Highly Efficient Electromagnetic Wave Absorbing Materials
| [5] | Che R C, Peng L M, Duan X F, Chen Q, and Liang X L 2004 Adv. Mater. 16 401 | Microwave Absorption Enhancement and Complex Permittivity and Permeability of Fe Encapsulated within Carbon Nanotubes
| [6] | Sun H, Che R C, You X, Jiang Y S, Yang Z B, Deng J, Qiu L B, and Peng H S 2014 Adv. Mater. 26 8120 | Cross-Stacking Aligned Carbon-Nanotube Films to Tune Microwave Absorption Frequencies and Increase Absorption Intensities
| [7] | Liu Q H, Cao Q, Bi H, Liang C Y, Yuan K P, She W, Yang Y J, and Che R C 2016 Adv. Mater. 28 486 | CoNi@SiO2 @TiO2 and CoNi@Air@TiO2 Microspheres with Strong Wideband Microwave Absorption
| [8] | Zhang X, Yan F, Zhang S, Yuan H R, Zhu C L, Zhang X T, and Chen Y J 2018 ACS Appl. Mater. & Interfaces 10 24920 | Hollow N-Doped Carbon Polyhedron Containing CoNi Alloy Nanoparticles Embedded within Few-Layer N-Doped Graphene as High-Performance Electromagnetic Wave Absorbing Material
| [9] | Sultanov F, Daulbayev C, Bakbolat B, and Daulbayev O 2020 Adv. Colloid Interface Sci. 285 102281 | Advances of 3D graphene and its composites in the field of microwave absorption
| [10] | Zhi D D, Li T, Li J Z, Ren H S, and Meng F B 2021 Compos. Part B 211 108642 | A review of three-dimensional graphene-based aerogels: Synthesis, structure and application for microwave absorption
| [11] | Hu C G, Mou Z Y, Lu G W, Chen N, Dong Z L, Hu M J, and Qu L T 2013 Phys. Chem. Chem. Phys. 15 13038 | 3D graphene–Fe3O4 nanocomposites with high-performance microwave absorption
| [12] | Ren S, Yu Q, Yu X H, Rong P, Jiang L Y, and Jiang J C 2020 Sci. Chin. Mater. 63 903 | Graphene-supported metal single-atom catalysts: a concise review
| [13] | Zhang L L, Liu D B, Muhammad Z, Wan F, Xie W, Wang Y J, Song L, Niu Z Q, and Chen J 2019 Adv. Mater. 31 1903955 | Single Nickel Atoms on Nitrogen‐Doped Graphene Enabling Enhanced Kinetics of Lithium–Sulfur Batteries
| [14] | Zhang X C, Shi Y N, Xu J, Ouyang Q Y, Zhang X, Zhu C L, Zhang X L, and Chen Y J 2022 Nano-Micro Lett. 14 27 | Identification of the Intrinsic Dielectric Properties of Metal Single Atoms for Electromagnetic Wave Absorption
| [15] | Wu L Z, Shu R W, Zhang J B, and Chen X T 2022 J. Colloid Interface Sci. 608 1212 | Synthesis of three-dimensional porous netlike nitrogen-doped reduced graphene oxide/cerium oxide composite aerogels towards high-efficiency microwave absorption
| [16] | Cheng Y, Zhao S Y, Li H B, He S, Veder J P, Johannessen B, Xiao J P, Lu S F, Pan J, Chisholm M F, Yang S Z, Liu C, Chen J G, and Jiang S P 2019 Appl. Catal. B: Environ. 243 294 | Unsaturated edge-anchored Ni single atoms on porous microwave exfoliated graphene oxide for electrochemical CO2
| [17] | Zhao H B, Cheng J B, Zhu J Y, and Wang Y Z 2019 J. Mater. Chem. C 7 441 | Ultralight CoNi/rGO aerogels toward excellent microwave absorption at ultrathin thickness
| [18] | Cao F H, Yan F, Xu J, Zhu C L, Qi L H, Li C Y, and Chen Y J 2021 Carbon 174 79 | Tailing size and impedance matching characteristic of nitrogen-doped carbon nanotubes for electromagnetic wave absorption
| [19] | Huang X G, Yu G Y, Zhang Y K, Zhang M J, and Shao G F 2021 Chem. Eng. J. 426 131894 | Design of cellular structure of graphene aerogels for electromagnetic wave absorption
| [20] | Zhang K C, Gao X B, Zhang Q, Li T P, Chen H, and Chen X F 2017 J. Alloys Compd. 721 268 | Synthesis, characterization and electromagnetic wave absorption properties of asphalt carbon coated graphene/magnetic NiFe2O4 modified multi-wall carbon nanotube composites
| [21] | Xu J, Zhang X, Zhao Z B, Yuan H R, Zhang S, Zhu C L, Zhang X T, and Chen Y J 2020 Carbon 159 357 | N-doped reduced graphene oxide aerogels containing pod-like N-doped carbon nanotubes and FeNi nanoparticles for electromagnetic wave absorption
| [22] | Yuan C Z, Zhan L Y, Liu S J, Chen F, Lin H J, Wu X L, and Chen J R 2020 Inorg. Chem. Front. 7 1719 | Semi-sacrificial template synthesis of single-atom Ni sites supported on hollow carbon nanospheres for efficient and stable electrochemical CO2 reduction
| [23] | Zhang X C, Zhang X, Yuan H R, Li K Y, Ouyang Q Y, Zhu C L, Zhang S, and Chen Y J 2020 Chem. Eng. J. 383 123208 | CoNi nanoparticles encapsulated by nitrogen-doped carbon nanotube arrays on reduced graphene oxide sheets for electromagnetic wave absorption
| [24] | Pandey R, Tekumalla S, and Gupta M 2019 J. Alloys Compd. 770 473 | Enhanced (X-band) microwave shielding properties of pure magnesium by addition of diamagnetic titanium micro-particulates
| [25] | Xu D W, Yang S, Chen P, Yu Q, Xiong X H, and Wang J 2019 Carbon 146 301 | Synthesis of magnetic graphene aerogels for microwave absorption by in-situ pyrolysis
| [26] | Liu J W, Che R C, Chen H J, Zhang F, Xia F, Wu Q S, and Wang M 2012 Small 8 1214 | Microwave Absorption Enhancement of Multifunctional Composite Microspheres with Spinel Fe3O4 Cores and Anatase TiO2 Shells
| [27] | Che R C, Zhi C Y, Liang C Y, and Zhou X G 2006 Appl. Phys. Lett. 88 033105 | Fabrication and microwave absorption of carbon nanotubes∕CoFe2O4 spinel nanocomposite
| [28] | Wu Z C, Pei K, Xing L S, Yu X F, You W B, and Che R C 2019 Adv. Funct. Mater. 29 1901448 | Enhanced Microwave Absorption Performance from Magnetic Coupling of Magnetic Nanoparticles Suspended within Hierarchically Tubular Composite
| [29] | Huang Y W, Wang Y J, Wei S C, Liang Y, Huang W, Wang B, and Xu B S 2019 Int. J. Mod. Phys. B 33 1940055 | Preparation of graphene/Fe3 O4 /Ni electromagnetic microwave absorbing nano-composite materials
| [30] | Chen T T, Deng F, Zhu J, Chen C F, Sun G B, Ma S L, and Yang X J 2012 J. Mater. Chem. 22 15190 | Hexagonal and cubic Ni nanocrystals grown on graphene: phase-controlled synthesis, characterization and their enhanced microwave absorption properties
| [31] | Ma T, Yuan M W, Islam S M, Li H F, Ma S L, Sun G B, and Yang X J 2016 J. Alloys Compd. 678 468 | FeNi3 alloy nanocrystals grown on graphene: Controllable synthesis, in-depth characterization and enhanced electromagnetic performance
| [32] | Yin P F, Deng Y, Zhang L M, Wu W J, Wang J, Feng X, Sun X Y, Li H Y, and Tao Y 2018 Ceram. Int. 44 20896 | One-step hydrothermal synthesis and enhanced microwave absorption properties of Ni0.5Co0.5Fe2O4/graphene composites in low frequency band
| [33] | Wang D T, Wang X C, Zhang X, Yuan H R, and Chen Y J 2020 Chin. Phys. Lett. 37 045201 | Tunable Dielectric Properties of Carbon Nanotube@Polypyrrole Core-Shell Hybrids by the Shell Thickness for Electromagnetic Wave Absorption*
| [34] | Tang J M, Liang N, Wang L, Li J, Tian G, Zhang D, Feng S H, and Yue H J 2019 Carbon 152 575 | Three-dimensional nitrogen-doped reduced graphene oxide aerogel decorated with Ni nanoparticles with tunable and unique microwave absorption
| [35] | Xu J, Liu M J, Zhang X C, Li B, Zhang X, Zhang X L, Zhu C L, and Chen Y J 2022 Appl. Phys. Rev. 9 011402 | Atomically dispersed cobalt anchored on N-doped graphene aerogels for efficient electromagnetic wave absorption with an ultralow filler ratio
| [36] | Chen J P, Jia H, Liu Z, Kong Q Q, Hou Z H, Xie L J, Sun G H, Zhang S C, and Chen C M 2020 Carbon 164 59 | Construction of C-Si heterojunction interface in SiC whisker/reduced graphene oxide aerogels for improving microwave absorption
| [37] | Liu B, Li J H, Wang L F, Ren J H, and Xu Y F 2017 Compos. Part A 97 141 | Ultralight graphene aerogel enhanced with transformed micro-structure led by polypyrrole nano-rods and its improved microwave absorption properties
| [38] | Cai Z X, Su L, Wang H J, Niu M, Gao H F, Lu D, and Li M Z 2020 ACS Appl. Mater. & Interfaces 12 8555 | Hydrophobic SiC@C Nanowire Foam with Broad-Band and Mechanically Controlled Electromagnetic Wave Absorption
| [39] | Cai Z X, Su L, Wang H J, Niu M, Tao L T, Lu D, Xu L, Li M Z, and Gao H F 2021 ACS Appl. Mater. & Interfaces 13 16704 | Alternating Multilayered Si3 N4 /SiC Aerogels for Broadband and High-Temperature Electromagnetic Wave Absorption up to 1000 °C