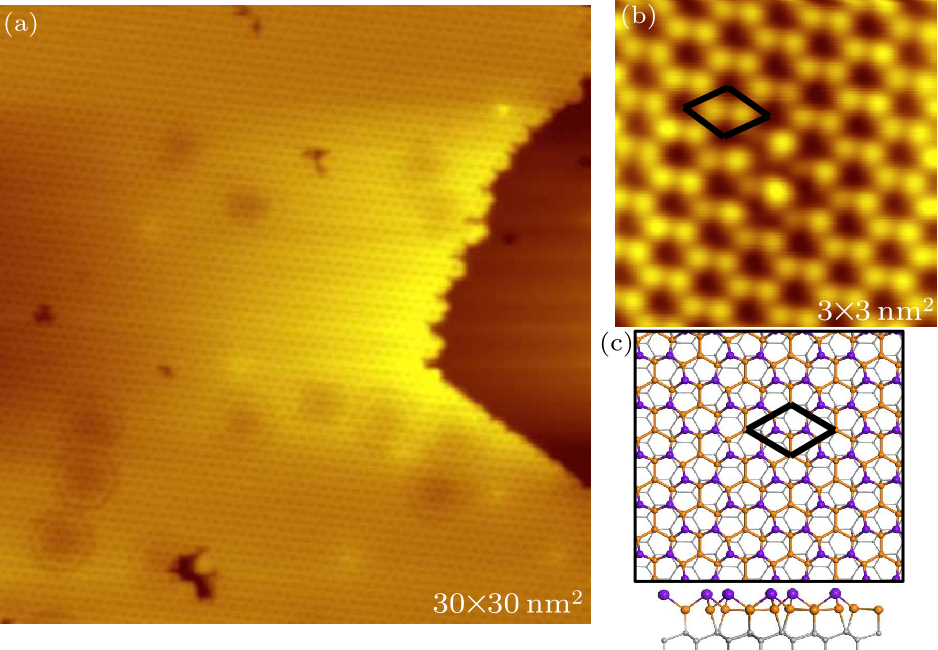
Fig. 1. STM image and structural model of clean Sn$_{2}$Bi on Si(111). (a) A typical topography image of a clean Sn$_{2}$Bi $2 \times 2$ surface (tip bias 2 V, tunneling current 100 pA). (b) Zoom-in image of the surface of (a) (tip bias $-1$ V, tunneling current 90 pA). The black rhombuses represent one unit cell. (c) Top and side views of the structural model of Sn$_{2}$Bi. Each unit cell consists of four Sn atoms and two upper-bulked Bi atoms. The gray balls are silicon atoms. The Sn atoms and Bi atoms are represented by yellow and purple balls, respectively.
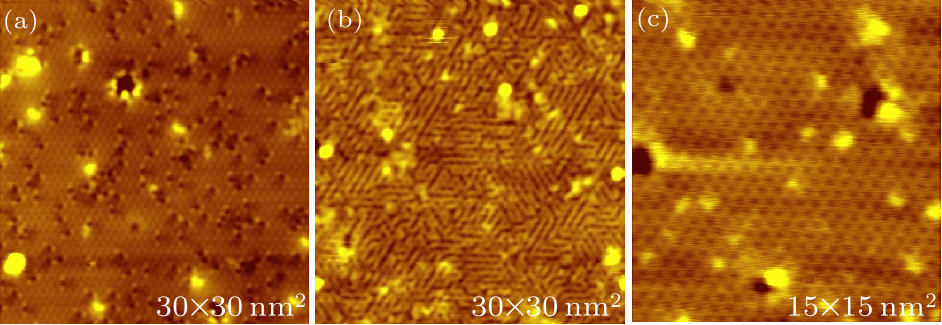
Fig. 2. STM images of (a) a clean monolayer Sn$_{2}$Bi after a small hydrogen exposure, (b) after a large hydrogen exposure and (c) after dehydrogenation. The scanning parameters of (a)–(c) are the same: tip bias $-2$ V and tunneling current 100 pA.
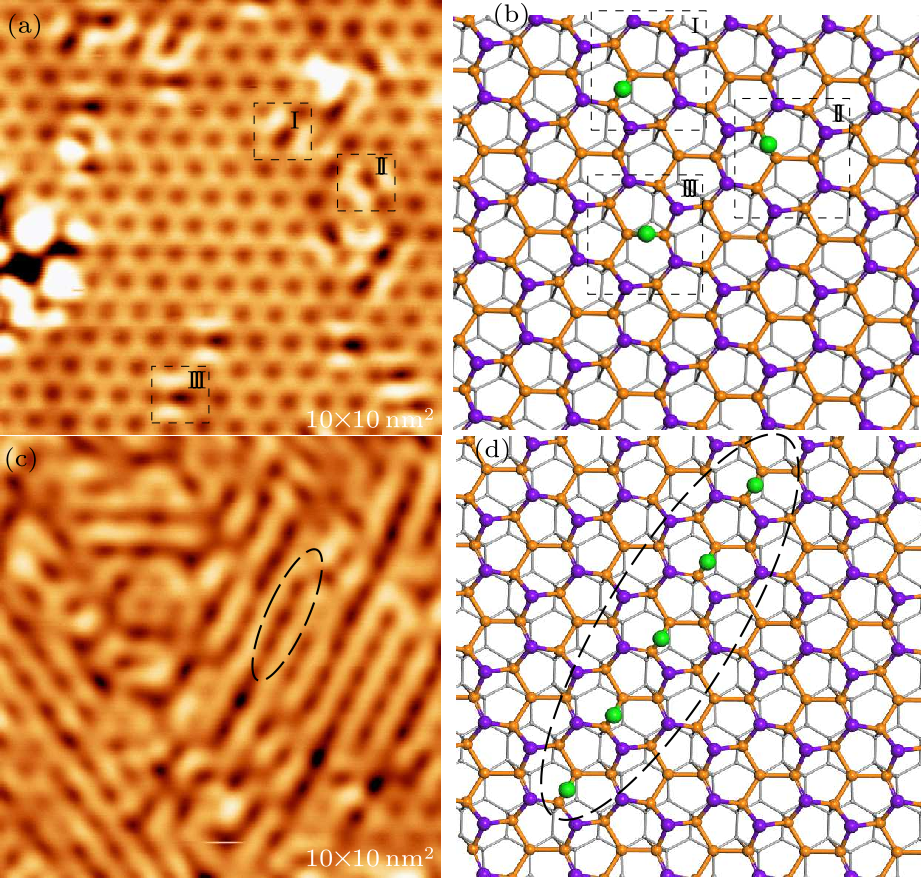
Fig. 3. STM image and structural model of the Sn$_{2}$Bi after hydrogenation with a small and fully hydrogen exposure. (a) A high-resolution STM topography image of Sn$_{2}$Bi with a small hydrogen exposure (tip bias $-2$ V, tunneling current 100 pA). (b) Structural model of (a). The green balls represent hydrogen atoms. (c) A high-resolution STM image of the Sn$_{2}$Bi after fully hydrogenated (tip bias $-2$ V, tunneling current 100 pA). (d) Gully structure models formed by the adsorption of hydrogen atoms in one of the three directions.
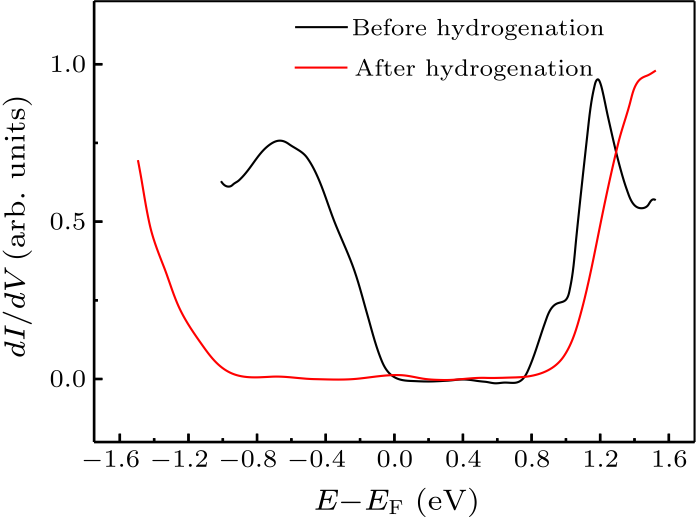
Fig. 4. STS results of Sn$_{2}$Bi before (black curve) and after (red curve) hydrogenation.
| [1] | Christmann K 1988 Surf. Sci. Rep. 9 1 | Interaction of hydrogen with solid surfaces
| [2] | Kraus A, Hanbucken M, Neddermeyer H 2002 Anal. Bioanal. Chem. 374 688 | Study by scanning tunneling microscopy of hydrogen adsorption and desorption on Si(111)7×7 at room temperature and at high temperature
| [3] | Mayne A J, Riedel D, Dujardin G 2006 Prog. Surf. Sci. 81 1 | Atomic-scale studies of hydrogenated semiconductor surfaces
| [4] | Balog R, Jorgensen B, Wells J, Laegsgaard E, Hofmann P, Hornekaer L 2009 J. Am. Chem. Soc. 131 8744 | Atomic Hydrogen Adsorbate Structures on Graphene
| [5] | Qiu J, Fu H, Xu Y, Oreshkin A I, Shao T, Li H, Meng S, Wu K 2015 Phys. Rev. Lett. 114 126101 | Ordered and Reversible Hydrogenation of Silicene
| [6] | Qiu J L, Fu H X, Xu Y, Zhou Q, Meng S, Li H, Wu K H 2015 ACS Nano 9 11192 | From Silicene to Half-Silicane by Hydrogenation
| [7] | Boland J J 1991 Surf. Sci. 244 1 | The importance of structure and bonding in semiconductor surface chemistry: hydrogen on the Si(111)-7 × 7 surface
| [8] | Balog R, Jorgensen B, Nilsson L, Andersen M, Rienks E, Bianchi M, Fanetti M, Laegsgaard E, Baraldi A, Lizzit S, Sljivancanin Z, Besenbacher F, Hammer B, Pedersen T G, Hornekaer L 2010 Nat. Mater. 9 315 | Bandgap opening in graphene induced by patterned hydrogen adsorption
| [9] | Sofo J O, Barber G D 2007 Phys. Rev. B 75 153401 | Graphane: A two-dimensional hydrocarbon
| [10] | Bobrov K, Mayne A, Comtet G, Hellner L 2003 Phys. Rev. B 68 195416 | Atomic-scale visualization and surface electronic structure of the hydrogenated diamond surface
| [11] | Freitas R R Q, Rivelino R, de Brito Mota F, de Castilho C M C, Gueorguiev G K 2015 J. Phys. Chem. C 119 23599 | Topological Insulating Phases in Two-Dimensional Bismuth-Containing Single Layers Preserved by Hydrogenation
| [12] | Fukuda T, Nakayama H 2006 Surf. Sci. 600 2443 | Scanning tunneling microscopy study on initial stage of atomic hydrogen adsorption on the Si(111) 7×7 surface
| [13] | Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Firsov A A 2004 Science 306 666 | Electric Field Effect in Atomically Thin Carbon Films
| [14] | Feng B J, Ding Z J, Meng S, Yao Y G, He X Y, Cheng P, Wu K H 2012 Nano Lett. 12 3507 | Evidence of Silicene in Honeycomb Structures of Silicon on Ag(111)
| [15] | Zhu F F, Chen W J, Xu Y, Gao C L, Guan D D, Liu C H, Qian D, Jia J F 2015 Nat. Mater. 14 1020 | Epitaxial growth of two-dimensional stanene
| [16] | Gou J, Kong L, Li H, Zhong Q, Li W, Cheng P, Chen L and Wu K 2017 Phys. Rev. Mater. 1 054004 | Strain-induced band engineering in monolayer stanene on Sb(111)
| [17] | Deng J, Xia B, Ma X, Chen H, Shan H, Zhai X, Li B, Xu Y and Duan W 2018 Nat. Mater. 17 1081 | Epitaxial growth of ultraflat stanene with topological band inversion
| [18] | Gou J, Xia B, Li H, Wang X, Kong L, Cheng P, Li H, Zhang W, Qian T, Ding H, Xu Y, Duan W, Chen L 2018 Phys. Rev. Lett. 121 126801 | Binary Two-Dimensional Honeycomb Lattice with Strong Spin-Orbit Coupling and Electron-Hole Asymmetry
| [19] | Ding Y and Wang Y 2019 Appl. Phys. Lett. 114 073103 | Stabilizing the isolated Sn 2 Bi nanosheet and tailoring its electronic structure by chemical functionalization: A computational study
| [20] | Vishkayi S I and Tagani M B 2020 J. Appl. Phys. 127 014302 | Tuning electronic and optical properties of free-standing Sn 2 Bi monolayer stabilized by hydrogenation
| [21] | Li T, Yu J, Zhang B and Sun Q 2020 Nano Energy 67 104283 | The ultralow thermal conductivity and ultrahigh thermoelectric performance of fluorinated Sn2Bi sheet in room temperature
| [22] | Gou J, Kong L J, Li W B, Sheng S X, Li H, Meng S, Cheng P, Chen L 2018 Phys. Chem. Chem. Phys. 20 20188 | Scanning tunneling microscopy investigations of unoccupied surface states in two-dimensional semiconducting β-√3 × √3-Bi/Si(111) surface