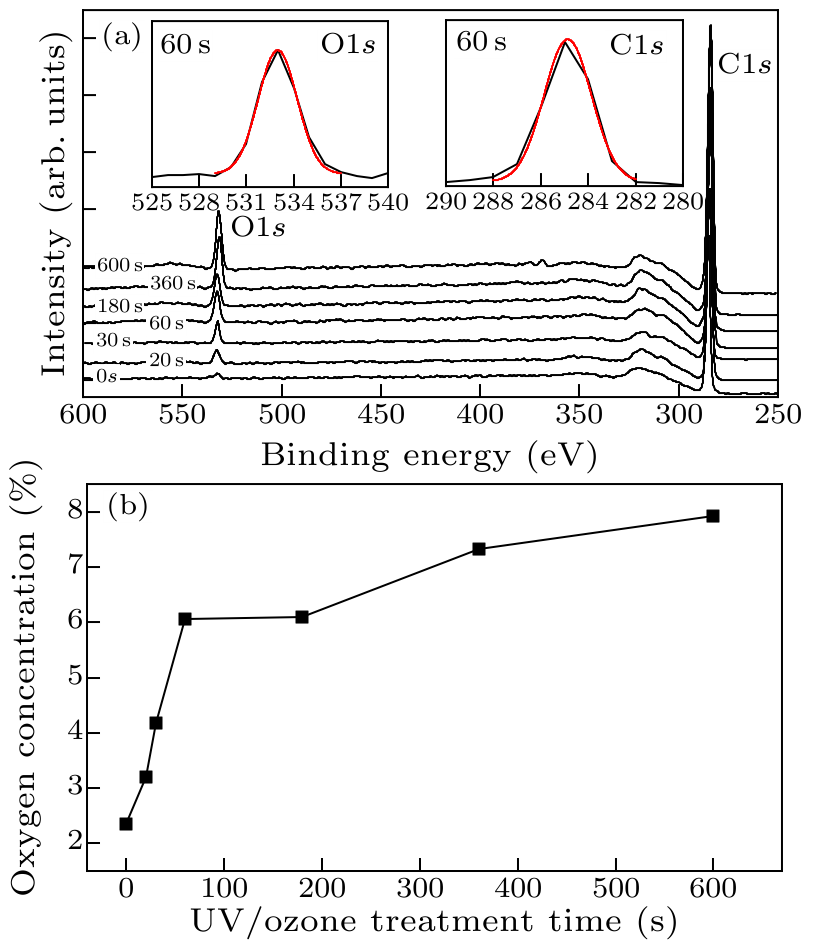
Fig. 1. (a) XPS spectra of scan over a wide range of binding energy of the H-diamond surfaces before (0 s) and after treated by UV/ozone for 20–600 s. The insets are the high-resolution scans of O $1s$ and C $1s$ of the surface treated for 60 s. (b) The content of adsorbed oxygen on diamond surface as a function of UV/ozone treatment time.
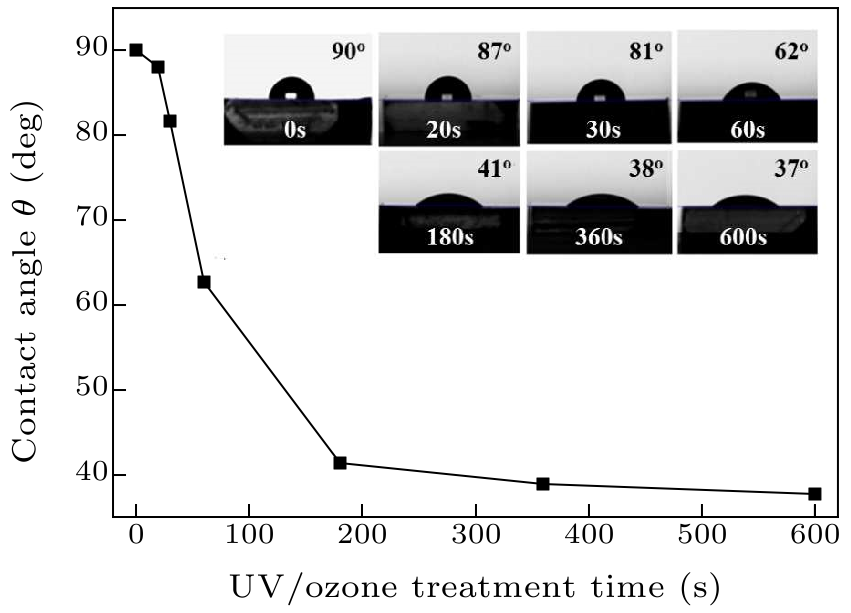
Fig. 2. Variation of contact angle for the UV/ozone treated H-diamond as a function of treatment time. The insets are the photographs of water droplets on the surfaces treated by UV/ozone for different times.
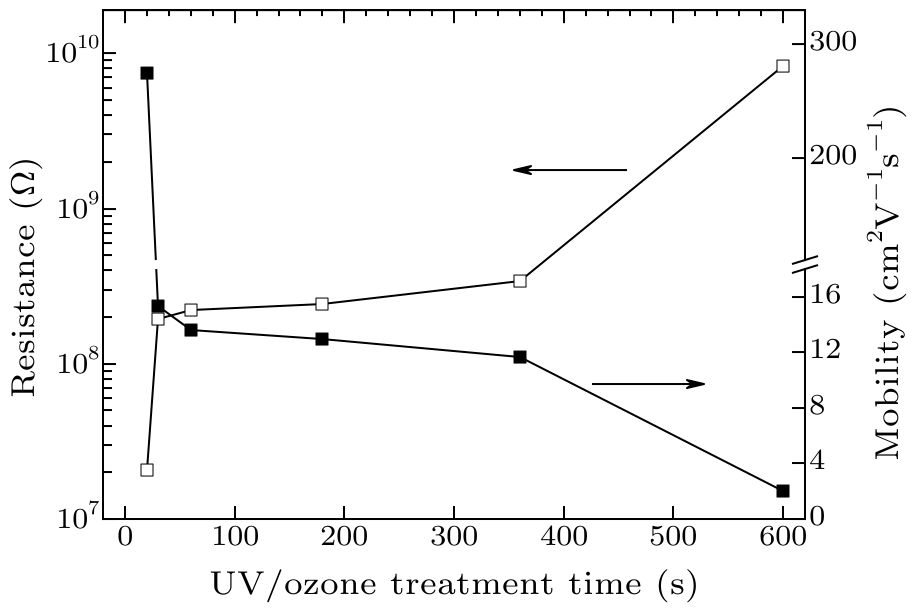
Fig. 3. Resistance and Hall mobility of the UV/ozone treated H-terminated diamonds as a function of treatment time.
| [1] | Nemanich R J, Carlisle J A, Hirata A and Haenen K 2014 MRS Bull. 39 490 | CVD diamond—Research, applications, and challenges
| [2] | Koizumi S, Umezawa H, Pernot J and Suzuki M 2018 Power Electronics Device Applications of Diamond Semiconductors (Amsterdam: Woodhead Publishing) |
| [3] | Nebel C and Ristein J 2003 Thin-Film Diamond I: Part of the Semiconductors and Semimetals Series (Amsterdam: Elsevier Academic Press) |
| [4] | Kawarada H 2012 Jpn. J. Appl. Phys. 51 090111 | High-Current Metal Oxide Semiconductor Field-Effect Transistors on H-Terminated Diamond Surfaces and Their High-Frequency Operation
| [5] | Satoshi K, Kenji W, Masataka H and Hisao K 2001 Science 292 1899 | Ultraviolet Emission from a Diamond pn Junction
| [6] | Shur M, Gelmont B and AsifKhan M 1996 J. Electron. Mater. 25 777 | Electron mobility in two-dimensional electron gas in AIGaN/GaN heterostructures and in bulk GaN
| [7] | Nebel C, Sauerer C, Ertl F, Stutzmann M and Jackman R 2001 Appl. Phys. Lett. 79 4541 | Hydrogen-induced transport properties of holes in diamond surface layers
| [8] | Kazushi H, Sadanori Y, Hideyo O and Koji K 1996 Appl. Phys. Lett. 68 376 | Study of the effect of hydrogen on transport properties in chemical vapor deposited diamond films by Hall measurements
| [9] | Maier F, Riedel M, Mantel B, Ristein J and Ley L 2000 Phys. Rev. Lett. 85 3472 | Origin of Surface Conductivity in Diamond
| [10] | Crawford K G, Qi D and McGlynn J 2018 Sci. Rep. 8 3342 | Thermally Stable, High Performance Transfer Doping of Diamond using Transition Metal Oxides
| [11] | Kasu M 2017 Jpn. J. Appl. Phys. 56 01AA01 | Diamond field-effect transistors for RF power electronics: Novel NO 2 hole doping and low-temperature deposited Al 2 O 3 passivation
| [12] | Oing D, Geller M, Lorke A and Wöhrl N 2019 Diamond Relat. Mater. 97 107450 | Tunable carrier density and high mobility of two-dimensional hole gases on diamond: The role of oxygen adsorption and surface roughness
| [13] | Hu X J and Li N 2013 Chin. Phys. Lett. 30 088102 | Oxygen Ion Implantation Enhanced Silicon-Vacancy Photoluminescence and n-Type Conductivity of Ultrananocrystalline Diamond Films
| [14] | Torrengo S and Minati L 2009 Diamond Relat. Mater. 18 804 | XPS and UPS investigation of the diamond surface oxidation by UV irradiation
| [15] | Kulisch W, Popov C, Gilliland D and Ceccone G 2010 Surf. Interface Anal. 42 1152 | Investigation of the UV/O3 treatment of ultrananocrystalline diamond films
| [16] | López G P, Castner D G and Ratner B D 1991 Surf. Interface Anal. 17 267 | XPS O 1s binding energies for polymers containing hydroxyl, ether, ketone and ester groups
| [17] | Kawarada H, Sasaki H and Sato A 1995 Phys. Rev. B 52 11351 | Scanning-tunneling-microscope observation of the homoepitaxial diamond (001) 2×1 reconstruction observed under atmospheric pressure
| [18] | Sakai T, Song K S, Kanazawa H and Nakamura Y 2003 Diamond Relat. Mater. 12 1971 | Ozone-treated channel diamond field-effect transistors
| [19] | Ma Z C, Gao N, Cheng S H, Liu J S, Yang M C, Wang P, Feng Z Y, Wang Q L and Li H D 2020 Chin. Phys. Lett. 37 046801 | Wettability and Surface Energy of Hydrogen- and Oxygen-Terminated Diamond Films
| [20] | Yang Y Z, Li H D, Cheng S H, Zou G T, Wang C X and Lin Q 2014 Chem. Commun. 50 2900 | Robust diamond meshes with unique wettability properties
| [21] | Yu X X, Zhou J J, Zhang S, Cao Z Y, Kong Y C and Chen T S 2019 Appl. Phys. Lett. 115 192102 | High frequency H-diamond MISFET with output power density of 182 mW/mm at 10 GHz
| [22] | Yuan X X, Gao N, Gao X, Qiu D C, Xu R, Sun Z L, Jiang Z G, Liu J S and Li H D 2019 Sens. Actuators B 281 830 | Nanopyramid boron-doped diamond electrode realizing nanomolar detection limit of 4-nonylphenol
| [23] | Strobel P, Riedel M, Ristein J and Ley L 2004 Nature 430 439 | Surface transfer doping of diamond
| [24] | Hirama K, Takayanagi H, Yamauchi S, Yang J H and Kawarada H 2008 Appl. Phys. Lett. 92 112107 | Spontaneous polarization model for surface orientation dependence of diamond hole accumulation layer and its transistor performance