**Corresponding author. Email: fanghaiping@sinap.ac.cn; gsshi@shu.edu.cn
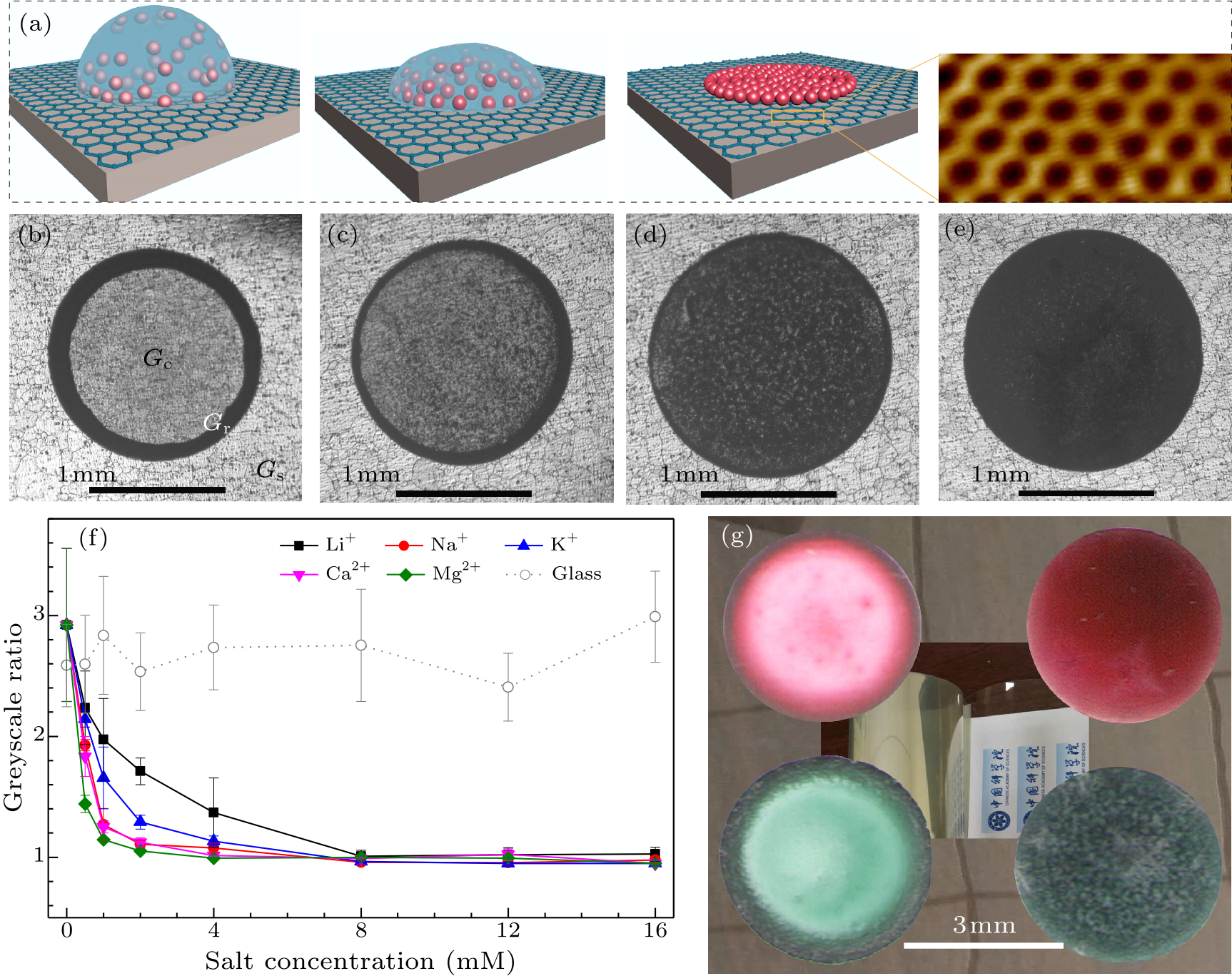
Fig. 1. Control of the deposition patterns of suspended matters by cations. (a) A schematic of how cations in a drop (blue hemisphere) determine the deposition of polystyrene microspheres (red beads). The inset shows an atomically resolved scanning tunneling microscopy (STM) image of a graphene lattice. (b)–(e) Optical microscopy images of particle patterns on graphene after evaporation of drops of suspension or mixture with different salt concentrations (0 mM, 2.0 mM, 4.0 mM, and 8.0 mM, respectively). (f) Greyscale ratios (GR = $\frac{G_{\rm c}-G_{\rm s}}{G_{\rm r}-G_{\rm s}}$) of patterns of the deposited matters on graphene (solid lines) and glass (dotted line) substrate. (g) Photographs of patterns on a PET film after evaporation of drops of mixtures of acid red 1 (upper) and acid blue 25 (lower) solutions with 0 mM (left) and 16.0 mM (right) NaCl, respectively.
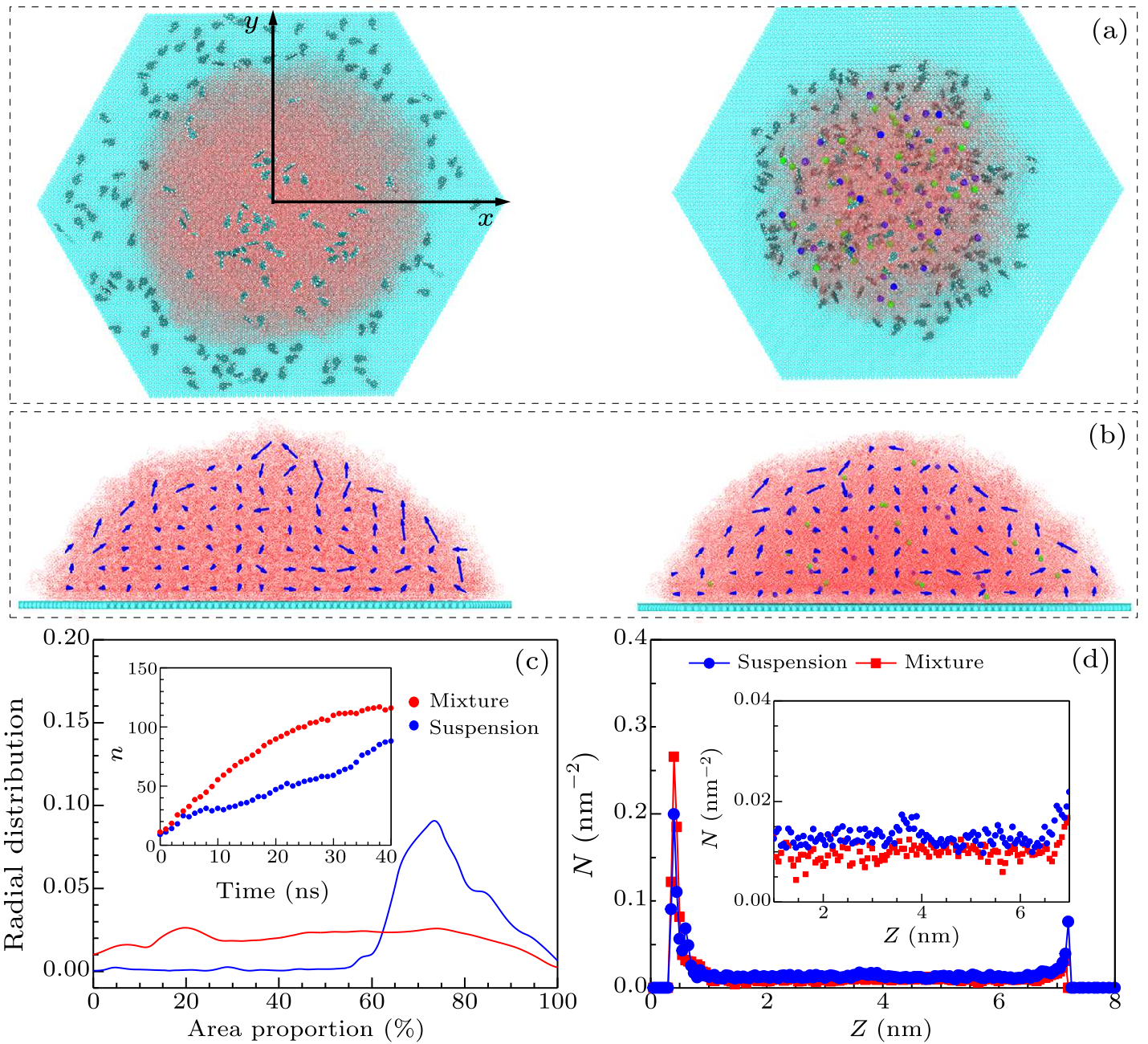
Fig. 2. Molecular dynamic simulations of the styrene molecules in drops of suspension or mixture on graphene sheets. (a) Top views of the drops of suspension and mixture on graphene during evaporation. The cyan, red, blue, green, and white spheres represent C, O, Na, Cl, H atoms, respectively. (b) Flow rate distributions in drop suspensions (without NaCl, left) or mixtures (with NaCl, right). (c) Radial distribution for the first layer ($ < $1 nm) of styrene molecules from the center of the drops. The inset plots the number $n$ of styrene molecules adsorbed on the surface ($n$) with time. (d) Density distribution of styrene molecules per square ($N$) along the $Z$ direction in the drops of suspension (blue) or mixture (red).
| [1] | Deegan R D, Bakajin O, Dupont T F et al 1997 Nature 389 827 | Capillary flow as the cause of ring stains from dried liquid drops
| [2] | Larson R G 2017 Nature 550 466 | Twenty years of drying droplets
| [3] | Minemawari H, Yamada T, Matsui H et al 2011 Nature 475 364 | Inkjet printing of single-crystal films
| [4] | Yunker P J, Still T, Lohr M A et al 2011 Nature 476 308 | Suppression of the coffee-ring effect by shape-dependent capillary interactions
| [5] | 2014 Nature 515 166 | Controlling the coffee-ring effect
| [6] | Han W and Lin Z 2012 Angew. Chem. Int. Ed. 51 1534 | Learning from “Coffee Rings”: Ordered Structures Enabled by Controlled Evaporative Self-Assembly
| [7] | Zhang Z, Zhang X, Xin Z et al 2013 Adv. Mater. 25 6714 | Controlled Inkjetting of a Conductive Pattern of Silver Nanoparticles Based on the Coffee-Ring Effect
| [8] | Diao Y, Tee B C K, Giri G et al 2013 Nat. Mater. 12 665 | Solution coating of large-area organic semiconductor thin films with aligned single-crystalline domains
| [9] | Devineau S, Anyfantakis M, Marichal L et al 2016 J. Am. Chem. Soc. 138 11623 | Protein Adsorption and Reorganization on Nanoparticles Probed by the Coffee-Ring Effect: Application to Single Point Mutation Detection
| [10] | Liu G L, Kim J, Lu Y et al 2006 Nat. Mater. 5 27 | Optofluidic control using photothermal nanoparticles
| [11] | Sempels W, De Dier R, Mizuno H et al 2013 Nat. Commun. 4 1757 | Auto-production of biosurfactants reverses the coffee ring effect in a bacterial system
| [12] | Talbot E L, Yang L, Berson A et al 2014 ACS Appl. Mater. & Interfaces 6 9572 | Control of the Particle Distribution in Inkjet Printing through an Evaporation-Driven Sol–Gel Transition
| [13] | Bail R, Hong J Y and Chin B D 2018 RSC Adv. 8 11191 | Inkjet printing of blue phosphorescent light-emitting layer based on bis(3,5-di(9 H -carbazol-9-yl))diphenylsilane
| [14] | Gorr H M, Zueger J M and Barnard J A 2012 J. Phys. Chem. B 116 12213 | Characteristic Size for Onset of Coffee-Ring Effect in Evaporating Lysozyme-Water Solution Droplets
| [15] | Lei Y, Zhang X, Xu D et al 2018 J. Phys. Chem. Lett. 9 2380 | Dynamic “Scanning-Mode” Meniscus Confined Electrodepositing and Micropatterning of Individually Addressable Ultraconductive Copper Line Arrays
| [16] | Cui L, Zhang J, Zhang X et al 2012 ACS Appl. Mater. & Interfaces 4 2775 | Suppression of the Coffee Ring Effect by Hydrosoluble Polymer Additives
| [17] | Li Y, Yang Q, Li M et al 2016 Sci. Rep. 6 24628 | Rate-dependent interface capture beyond the coffee-ring effect
| [18] | Manos A and Damien B 2014 Angew. Chem. Int. Ed. 53 14077 | Dynamic Photocontrol of the Coffee-Ring Effect with Optically Tunable Particle Stickiness
| [19] | Seo C, Jang D, Chae J et al 2017 Sci. Rep. 7 500 | Altering the coffee-ring effect by adding a surfactant-like viscous polymer solution
| [20] | Anyfantakis M, Geng Z, Morel M et al 2015 Langmuir 31 4113 | Modulation of the Coffee-Ring Effect in Particle/Surfactant Mixtures: the Importance of Particle–Interface Interactions
| [21] | Tekin E, De Gans B J and Schubert U S 2004 J. Mater. Chem. 14 2627 | Ink-jet printing of polymers ? from single dots to thin film libraries
| [22] | Soltman D and Subramanian V 2008 Langmuir 24 2224 | Inkjet-Printed Line Morphologies and Temperature Control of the Coffee Ring Effect
| [23] | Yen T M, Fu X, Wei T et al 2018 Sci. Rep. 8 3157 | Reversing Coffee-Ring Effect by Laser-Induced Differential Evaporation
| [24] | Dugyala V R and Basavaraj M G 2014 Langmuir 30 8680 | Control over Coffee-Ring Formation in Evaporating Liquid Drops Containing Ellipsoids
| [25] | Larson R G 2012 Angew. Chem. Int. Ed. 51 2546 | Re-Shaping the Coffee Ring
| [26] | Liu L H , Zorn G, Castner D G et al 2010 J. Mater. Chem. 20 5041 | A simple and scalable route to wafer-size patterned graphene
| [27] | Shuping P, Yenny H, Xinliang F et al 2011 Adv. Mater. 23 2779 | Graphene as Transparent Electrode Material for Organic Electronics
| [28] | Novoselov K S, Fal'ko V I, Colombo L et al 2012 Nature 490 192 | A roadmap for graphene
| [29] | Esfandiar A, Radha B, Wang F C et al 2017 Science 358 511 | Size effect in ion transport through angstrom-scale slits
| [30] | Jain T, Rasera B C, Guerrero R J S et al 2015 Nat. Nanotechnol. 10 1053 | Heterogeneous sub-continuum ionic transport in statistically isolated graphene nanopores
| [31] | Wu X, Pei Y and Zeng X C 2009 Nano Lett. 9 1577 | B 2 C Graphene, Nanotubes, and Nanoribbons
| [32] | Stankovich S, Dikin D A, Dommett G H B et al 2006 Nature 442 282 | Graphene-based composite materials
| [33] | Yasaei P, Kumar B, Hantehzadeh R et al 2014 Nat. Commun. 5 4911 | Chemical sensing with switchable transport channels in graphene grain boundaries
| [34] | Secor E B, Lim S, Zhang H et al 2014 Adv. Mater. 26 4533 | Gravure Printing of Graphene for Large-area Flexible Electronics
| [35] | Shi G, Chen L, Yang Y et al 2018 Nat. Chem. 10 776 | Two-dimensional Na–Cl crystals of unconventional stoichiometries on graphene surface from dilute solution at ambient conditions
| [36] | Chen L, Shi G, Shen J et al 2017 Nature 550 380 | Ion sieving in graphene oxide membranes via cationic control of interlayer spacing
| [37] | Shi G, Liu J, Wang C et al 2013 Sci. Rep. 3 3436 | Ion Enrichment on the Hydrophobic Carbon-based Surface in Aqueous Salt Solutions due to Cation-π Interactions
| [38] | Ma J C and Dougherty D A 1997 Chem. Rev. 97 1303 | The Cation−π Interaction
| [39] | Mahadevi A S and Sastry G N 2013 Chem. Rev. 113 2100 | Cation−π Interaction: Its Role and Relevance in Chemistry, Biology, and Material Science
| [40] | Shi G, Dang Y, Pan T et al 2016 Phys. Rev. Lett. 117 238102 | Unexpectedly Enhanced Solubility of Aromatic Amino Acids and Peptides in an Aqueous Solution of Divalent Transition-Metal Cations