| CROSS-DISCIPLINARY PHYSICS AND RELATED AREAS OF SCIENCE AND TECHNOLOGY |
 |
|
|
|
|
A High-Temperature $\beta$-Phase NaMnO$_{2}$ Stabilized by Cu Doping and Its Na Storage Properties |
| Li-Wei Jiang1,2, Ya-Xiang Lu1**, Yue-Sheng Wang1, Li-Lu Liu1,2, Xing-Guo Qi1,2, Cheng-Long Zhao1,2, Li-Quan Chen1, Yong-Sheng Hu1,2** |
1Key Laboratory for Renewable Energy, Beijing Key Laboratory for New Energy Materials and Devices, and Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190
2School of Physical Sciences, University of Chinese Academy of Sciences, Beijing 100049 |
|
| Cite this article: |
|
Li-Wei Jiang, Ya-Xiang Lu, Yue-Sheng Wang et al 2018 Chin. Phys. Lett. 35 048801 |
|
|
|
|
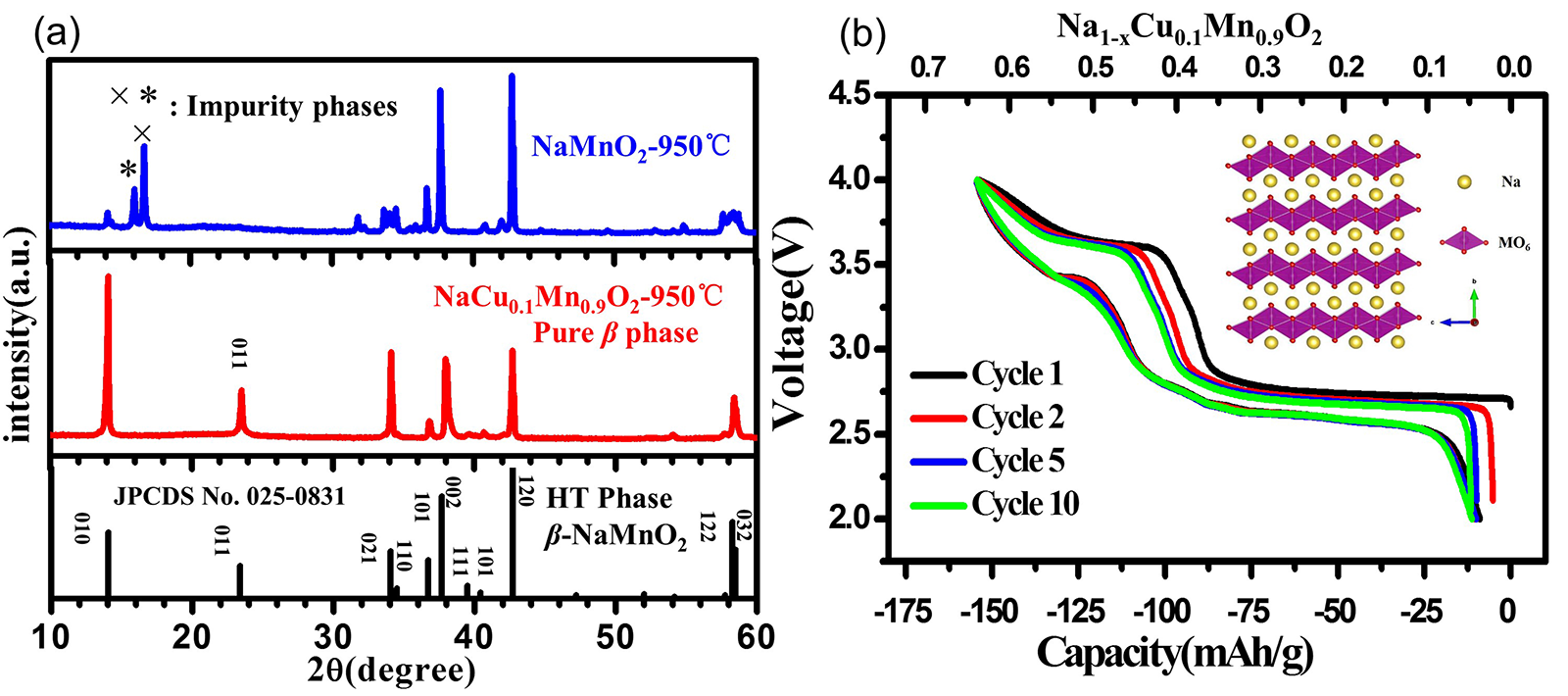
Abstract The high-temperature $\beta$-phase NaMnO$_{2}$ is a promising material for Na-ion batteries (NIBs) due to its high capacity and abundant resources. However, the synthesis of phase-pure $\beta$-NaMnO$_{2}$ is burdensome and cost-ineffective because it needs to be sintered under oxygen atmosphere at high temperature and followed by a quenching procedure. Here we first report that the pure $\beta$ phase can be stabilized by Cu-doping and easily synthesized by replacing a proportion of Mn with Cu via a simplified process including sintering in air and cooling to room temperature naturally. Based on the first-principle calculations, the band gap decreases from 0.7 eV to 0.3 eV, which indicates that the electronic conductivity can be improved by Cu-doping. The designed $\beta$-NaCu$_{0.1}$Mn$_{0.9}$O$_{2}$ is applied as cathode in NIBs, exhibiting an energy density of 419 Wh/kg and better performance in terms of rate capability and cycling stability than those in the undoped case.
|
|
Received: 01 March 2018
Published: 13 March 2018
|
|
| PACS: |
88.80.ff
|
(Batteries)
|
| |
82.47.Aa
|
(Lithium-ion batteries)
|
| |
71.20.-b
|
(Electron density of states and band structure of crystalline solids)
|
| |
68.55.Nq
|
(Composition and phase identification)
|
|
|
| Fund: Supported by the National Key Technologies R&D Program of China under Grant No 2016YFB0901500, and the National Nature Science Foundation of China under Grant Nos 51725206 and 51421002. |
|
|
|
| [1] | Mizushima K, Jones P C, Wiseman P J and Goodenough J B 1980 Mater. Res. Bull. 15 783 | | [2] | Arm M and Tarascon J M 2008 Nature 451 652 | | [3] | Zu C X and Li H 2011 Energy Environ. Sci. 4 2614 | | [4] | Su H, Jaffer S and Yu H 2016 Energy Storage Mater. 5 116 | | [5] | Slater M D, Kim D, Lee E and Johnson C S 2013 Adv. Funct. Mater. 23 947 | | [6] | Li Y, Lu Y, Zhao C, Hu Y, Titirici M, Li H, Huang X and Chen L 2017 Energy Storage Mater. 7 130 | | [7] | Kim S W, Seo D H, Ma X H, Ceder G and Kang K 2012 Adv. Energy Mater. 2 710 | | [8] | Komaba S, Murata W, Ishikawa T, Yabuuchi N, Ozeki T, Nakayama T, Ogata A, Gotoh K and Fujiwara K 2011 Adv. Funct. Mater. 21 3859 | | [9] | Li Y, Hu Y, Qi X, Rong X, Li H, Huang X and Chen L 2016 Energy Storage Mater. 5 191 | | [10] | Xu S, Wang Y, Ben L, Lyu Y, Song N, Yang Z, Li Y, Mu L, Yang H, Gu L, Hu Y, Li H, Cheng Z, Chen L and Huang X 2015 Adv. Energy Mater. 5 201501156 | | [11] | Zhao L, Zhao J, Hu Y S, Li H, Zhou Z, Arm, M and Chen L 2012 Adv. Energy Mater. 2 962 | | [12] | Yabuuchi, Naoaki, Kubota Kei, Dahbi Mouad and Komaba Shinichi 2013 Chem. Rev. 113 6552 | | [13] | Sun Y, Zhao L, Pan H, Lu X, Gu L, Hu Y S, Li H, Arm, M, Ikuhara Y, Chen L and Huang X 2013 Nat. Commun. 4 1870 | | [14] | Pan H, Lu X, Yu X, Hu Y S, Li H, Yang X Q and Chen L 2013 Adv. Energy Mater. 3 1186 | | [15] | Wang Y, Yu X Q, Xu S Y, Bai J, Xiao R J, Hu Y S, Li H, Yang X Q, Chen L Q and Huang X J 2013 Nat. Commun. 4 2365 | | [16] | Qi X, Wang Y, Jiang L, Mu L, Zhao C, Liu L, Hu Y S and Chen L 2016 Part. Part. Syst. Charact. 33 538 | | [17] | Rong X, Liu J, Hu E, Liu Y, Wang Y, Wu J, Yu X, Page K, Hu Y, Yang X, Chen L and Huang X 2018 Joule 2 125 | | [18] | Mu L, Xu S, Li Y, Hu Y, Li H, Chen L and Huang X 2015 Adv. Mater. 27 6928 | | [19] | Wang L, Lu Y H, Liu J, Xu M W, Cheng J G, Zhang D W and Goodenough J B 2013 Angew. Chem. Int. Ed. 52 1964 | | [20] | Wang Y, Mu L, Liu J, Yang Z, Yu X, Gu L, Hu Y S, Li H, Yang X, Chen L and Huang X 2015 Adv. Energy Mater. 5 1501005 | | [21] | Jian Z, Han W, Lu X, Yang H, Hu Y S, Zhou J, Zhou Z, Li J, Chen W, Chen D and Chen L 2013 Adv. Energy Mater. 3 156 | | [22] | Li Y, Yang Z, Xu S, Mu L, Gu L, Hu Y, Li H and Chen L 2015 Adv. Sci. 2 1500031 | | [23] | Xu S, Wu X, Li Y, Hu Y and Chen L 2014 Chin. Phys. B 23 118202 | | [24] | Mu L, Hu Y and Chen L 2015 Chin. Phys. B 24 038202 | | [25] | Billaud J, Cleíment R J, Armstrong A R, Canales V J, Rozier P, Grey C P and Bruce P G 2014 J. Am. Chem. Soc. 136 17243 | | [26] | Mendiboure A, Delmas C and Hagenmuller P 1971 Solid State Chem. 3 1 | | [27] | Velikokhatnyi O, Chang C and Kumta P 2003 J. Electrochem. Soc. 150 A1262 | | [28] | Kresse G and Furthmüller J 1996 Phys. Rev. B 54 11169 | | [29] | Shi S, Gao J, Liu Y, Zhao Y, Wu Q, Ju W, Ouyang C and Xiao R 2016 Chin. Phys. B 25 018212 | | [30] | Anisimov V, Zaanen J and Andersen O 1991 Phys. Rev. B 44 943 | | [31] | Perdew J, Burke K and Ernzerhof M 1996 Phys. Rev. Lett. 77 3865 | | [32] | Nolan M and Elliott S D 2006 Phys. Chem. Chem. Phys. 8 5350 | | [33] | Wang Z, Chen Y and Ouyang C 2014 Phys. Lett. A 378 2449 | | [34] | Ong S, Chevrier V and Ceder G 2011 Phys. Rev. B 83 075112 | | [35] | Cleímnt R, Middlemiss D, Seymour I, Ilott A and Grey C 2016 Chem. Mater. 28 8228 |
|
|
Viewed |
|
|
|
Full text
|
|
|
|
|
Abstract
|
|
|
|
|